Abstract
Tobacco dependence is an addiction with high rates of relapse, resulting in multiple quit attempts in individuals who are trying to stop smoking. How these multiple cycles of smoking and withdrawal contribute to nicotine dependence, long-term alterations in brain reward systems, and nicotine receptor regulation is unknown. Therefore, to evaluate how multiple exposures of nicotine and withdrawal periods modulate rewarding properties of nicotine, we used intracranial self-stimulation to measure alterations in the threshold of brain stimulation reward. In addition, we employed the conditioned place preference (CPP) paradigm to evaluate positive context conditioning following each withdrawal period and measured levels of neuronal nicotinic receptors in cortex, striatum, and hippocampus. We found that repeated nicotine exposure and withdrawal enhanced brain stimulation reward and reward sensitivity to acute injections of nicotine. This increased reward was reflected by enhanced CPP to nicotine. Chronic nicotine is known to up-regulate nAChRs (nicotinic acetylcholine receptors) and we found that this up-regulation was maintained for up to 8 days of withdrawal in the striatum and in the hippocampus, but not in the cortex, of animals exposed to multiple nicotine exposure and withdrawal periods. These results demonstrate that repeated exposures to nicotine, followed by withdrawal, induce a persistent increase in both brain reward function and sensitivity to the hedonic value of nicotine and long-lasting up-regulation of neuronal nicotinic receptors. Together, these data suggest that a continuing increase in brain reward function and enhanced sensitivity to nicotine reward following repeated withdrawal periods may be one reason why smokers relapse frequently.
Similar content being viewed by others
INTRODUCTION
Tobacco dependence is an addiction with high rates of relapse among those who try to quit. Between 60 and 90% of smokers relapse within the first year of quitting; thus, maintenance of smoking cessation following a single withdrawal is rare (Krall et al, 2002). As a result, individuals trying to quit often experience multiple cycles of smoking and withdrawal. Nicotine, the major addictive component of tobacco, alters reward systems and cognitive and psychomotor processes via its action on nicotinic acetylcholine receptors (nAChRs) in the nervous system (Changeux, 2010). Both intravenously self-administered and experimenter-administered nicotine can increase brain reward sensitivity measured by intracranial self-stimulation (ICSS) (Cryan et al, 2003a; Harrison et al, 2002; Kenny and Markou, 2006b). However, unlike other drugs of abuse (Kenny et al, 2003b, 2006a; Cryan et al, 2003b; Paterson et al, 2000), nicotine withdrawal is not consistently characterized by decreases in brain reward sensitivity (Johnson et al, 2008; Kenny and Markou, 2006b; Stoker et al, 2008), and in one case increased brain reward sensitivity lasted for up to 36 days in rats (Kenny and Markou, 2006b). More recently, conditioned withdrawal cues have been shown to directly amplify the incentive properties of cues associated with nicotine (Scott and Hiroi, 2010). Together, these studies suggest that nicotine withdrawal contributes to alterations in brain reward systems that may promote nicotine dependence.
The present study models the cycle of smoking relapse by using multiple nicotine exposure and withdrawal periods and tests the impact on brain reward systems in mice. We show that repeated episodes of nicotine exposure and withdrawal induce a persistent increase in brain reward sensitivity and increases the hedonic value of nicotine, which is associated with up-regulation of nAChRs in the striatum and hippocampus. These findings are clinically relevant to smoking behavior, and identify a novel conceptual framework for investigating nicotine dependence and understanding its treatment.
MATERIALS AND METHODS
Animals
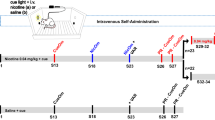
Male C57BL/6J mice (Taconic, Hudson, New York), 8 weeks of age and weighing ∼25–30 g, were used in all experiments. For the ICSS studies, mice were implanted with nicotine containing minipumps (NMP; 24 mg/kg/day) or saline minipumps (SMP). For the repeated nicotine exposure and withdrawal study, mice were implanted with 1, 2, or 3 minipumps and experienced 1, 2, or 3 withdrawal periods (WD), respectively. Cohorts of animals were as follows: Animals receiving 1NMP/1WD, n=16; 2NMPs/2WD, n=16; 3NMPs/3WD, n=8. Animals receiving 1SMP/1WD, n=7; 2SMPs/2WD, n=7; 3SMPs/3WD, n=7. For the spontaneous and precipitated withdrawal study, six mice were implanted with NMP using a dose of 40 mg/kg/day and eight mice were implanted with SMP. Subjects used in the ICSS experiments were housed individually.
For the conditioned place preference (CPP) studies, separate cohorts of animals were used for each nicotine exposure and withdrawal episode. For the control group, nine mice were implanted with SMP. For treatment groups (see Figure 4a), seven mice were implanted with NMP (24 mg/kg/day; 14 days) and were tested in the CPP paradigm following one withdrawal (1WD); six mice were implanted with two NMPs separated by a 2-week withdrawal and were tested in CPP following the second withdrawal (2WD); nine mice were implanted with three NMPs separated by two 2-week withdrawal periods and were tested in CPP following the third withdrawal period (3WD). Subjects used in the CPP experiment were housed in groups of four per cage. All subjects were tested during the light phase of the light/dark cycle and given ad libitum access to food and water. Subjects were treated in accordance with the National Institutes of Health guidelines, and approved by the University of Pennsylvania Animal Care and Use Committee. Experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Drugs
Doses of nicotine tartrate (Sigma-Aldrich, St Louis, MO) are reported as free base weight. For acute studies, nicotine solution was adjusted to pH 7.4 with NaOH, and administered subcutaneously (0.5 mg/kg). This dose of nicotine has been shown to induce nicotine CPP in mice (Grabus et al, 2006; Risinger and Oakes, 1995). The 24 mg/kg/day dose used for chronic nicotine treatments was based on studies that have shown nicotine tolerance as measured by antinociception and withdrawal effects (Matta et al, 2007). Of interest, this dose yields plasma levels of ∼0.3 μM (based on nicotine free base molecular weight) (Matta et al, 2007), a concentration similar to that observed in human smokers consuming an average of 17 cigarettes a day (plasma levels between 0.06 and 0.31 μM) (Matta et al, 2007). The rationale for the use of 40 mg/kg/day was based on previous mouse studies that have shown the need for higher doses to observe anhedonia/withdrawal effects using ICSS (Stoker et al, 2008, 2012).
Mecamylamine hydrochloride, a nonspecific nAChR antagonist with a slightly higher affinity for α3β4 receptors (Papke et al, 2001) (0, 3.0, 6.0 mg/kg; Sigma-Aldrich) was dissolved in saline and administered intraperitoneally. All drugs were injected in volumes of 0.1 ml/10 g body weight. Mecamylamine doses were chosen based on previous literature, demonstrating significant withdrawal effect in mice while failing to induce changes in saline-treated animals using ICSS (Stoker et al, 2008, 2012). Mecamylamine doses were administered according to a 3X3 within-subjects Latin square design.
Surgical Procedures
For all surgeries, mice were anesthetized with an isoflurane/oxygen vapor mixture (1–3%). Nicotine tartrate (24 or 40 mg/kg/day) was dissolved in sterile 0.9% saline solution and infused through subcutaneous osmotic minipumps for 14 days (model 2002, Alzet, Palo Alto, CA, USA). Nicotine solution in the osmotic minipumps was kept acidic at pH 4, for a slower degradation of nicotine overt time, since neutralized nicotine solutions are known to become unstable and degrade at a faster rate (Matta et al, 2007). Minipumps were inserted subcutaneously and placed parallel to the spine at shoulder level with the flow moderator directed away from the wound. The wound was closed with 7 mm stainless steel wound clips (Reflex, Cellpoint Scientific, Gaithersburg, MD, USA).
ICSS: Surgeries were performed as previously described (Ho et al, 2012). Briefly, bipolar stimulating electrodes (6 mm in length; 0.125 mm in diameter) were lowered into the medial forebrain bundle at the level of the lateral hypothalamus (coordinates AP: −1.9 mm from bregma; ML: ±0.8 mm; DV: −4.8 mm from skull surface; flat-skull position, according to Paxinos and Franklin (2003). Following implantation, animals were housed individually and allowed to recover for 1 week before ICSS training.
Experimental Procedures
For the ICSS procedure, mice were trained in operant chambers (Med Associates, St Albans, VT) using a modified discrete-trial current-threshold procedure (Markou and Koob, 1992). Current-intensity thresholds were used as a measure of reward. Each testing session was ∼45 min in duration. Response latencies were defined as the time that elapsed between the onset of the noncontingent stimulus and a positive response. The response latency for each test session was defined as the mean response latency of all trials. Baseline activity of brain reward systems remain stable following repeated exposures to ICSS (Markou and Koob, 1992). In the present studies, stable ICSS thresholds were defined as ⩽10% variation over a 3-day period, which were established after 10–14 days of training. To address how reward sensitivity post-nicotine exposure is affected in comparison to a drug-naive state, all data were normalized to the mean thresholds obtained for each animal before any nicotine treatment, following the example of Kenny and Markou (2006b). Following stabilization of ICSS thresholds, mice received either saline or nicotine containing minipumps for 2 weeks. Twenty-four hours after minipump removal, nicotine exposed mice experienced a natural withdrawal; these animals were further divided into two groups; one receiving acute nicotine injections (0.5 mg/kg) and the other saline. Following 4 days of acute injections, all animals experienced 1 week of nicotine abstinence. This paradigm was repeated two more times to compare the effects of multiple withdrawals. Mice receiving SMP followed the same cyclic paradigm but also received acute nicotine injections during the 4 days post-MP removal.
To evaluate the effect of nicotine in spontaneous withdrawal, mice were exposed to a higher concentration of nicotine (40 mg/kg/day for 14 days). ICSS thresholds were measured during the 5 days post-nicotine minipump extraction. To evaluate precipitated withdrawal, mecamylamine was administered intraperitoneally (0, 3.0, 6.0 mg/kg) on days 9, 11, and 13.
CPP Procedure
Mice were exposed to nicotine (24 mg/kg/day, 14 days) via osmotic minipumps. Twenty-four hours following minipump removal, mice were preconditioned in chambers (15.9 × 14.0 × 12.7 cm3), which had two distinct compartments (separated by a door). One compartment had vertical white and black walls with a stainless steel grid rod floor consisting of 0.31 cm rods placed on 0.78 cm centers. The other compartment had horizontal white and black stripes with a 0.625 × 0.625 cm2 stainless steel mesh floor (Med Associates). An unbiased design was used, as preliminary data indicated no preconditioned compartment preferences for the C57BL/6J strain. Mice were individually placed into the apparatus and allowed to roam freely between the two compartments for 900 s. Conditioning sessions took place 4 days after minipump removal, corresponding to the time point in the ICSS study when the nicotine injections showed a reduction in reward thresholds. Twice daily pairings, with a minimum of 5 h between conditioning sessions, occurred over the course of 4 days with a counterbalanced design. At 24 h following the last pairing, a post-conditioning test was performed, where animals had access to both sides of the apparatus. The time that the mice spent in each compartment was recorded for 15 min. CPP scores were expressed as the difference in the time spent (in seconds) in the saline-paired compartment and the time spent in the drug-paired compartment.
To parallel the ICSS studies, three groups of animals were tested in CPP using comparable nicotine exposure and withdrawal paradigms. Three groups of animals were tested following either one, two, or three exposures to nicotine and the equivalent number of withdrawal periods. Each group underwent a single CPP conditioning phase (days 4–7 of WD) that was carried out after the removal of their last nicotine MP (see Figure 4a for details). The saline groups were treated as one for statistical purposes for both behavior and binding studies (see below).
Receptor Binding
Tissues were harvested from animals immediately following CPP testing on day 8. Cortex, striatum (including caudate/putamen and nucleus accumbens), and hippocampus (including dentate gyrus, CA3, CA2, CA1, and subiculum) were dissected according to coordinates defining these regions in the Mouse Brain Atlas (Paxinos and Franklin, 2003). Cortex: saline group (n=3), 1WD (n=4), 2WD (n=5), 3WD (n=4); striatum, saline group (n=9), 1WD (n=4), 2WD (n=5), 3WD (n=9); hippocampus, saline group (n=6), 1WD (n=4), 2WD (n=5), 3WD (n=7). Samples were homogenized in 50 mM Tris–HCl (Sigma-Aldrich) buffer, pH 7.4 at 24°C, and centrifuged twice at 35 000 × g for 10 min in fresh buffer. Membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]epibatidine ([3H]EB; Perkin-Elmer, Boston, MA). This ligand was selected because of its extremely low nonspecific binding and its high-affinity binding to all heteromeric nAChRs. Incubations were performed in Tris buffer at pH 7.4 for 2 h at 24°C with [3H]EB. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass fiber filters (Brandel, Gaithersburg, MD) pre-treated with 0.5% polyethyleneimine (Sigma-Aldrich). Nonspecific binding was determined in the presence of 300 mM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding.
Statistics
All analyses were performed using GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA). Data were analyzed using one-way ANOVA with Newman–Keul's post hoc or two-way ANOVA with Bonferroni post hoc as appropriate. Statistical significance for all tests was set at α=0.05. For all ICSS data, with the exception of the mecamylamine treatment, threshold and response latency was expressed as percentage of baseline values obtained during the last three daily sessions before the first minipump implantation. To evaluate the effects of mecamylamine on ICSS performance, thresholds were expressed as percentage of the day preceding mecamylamine/vehicle administration.
RESULTS
Alterations in Brain Stimulation Reward During Chronic Nicotine Treatment and Withdrawal
We used ICSS to measure alterations in brain stimulation reward as this method has the main advantage of permitting a direct assessment of drug-induced changes in reward circuits. ICSS is a valuable technique to study how nicotine affects reward, given its capacity to discriminate between hedonic and anhedonic states. Additionally, once animals have acquired ICSS operant behavior, the same animals can be tested across time (Carlezon and Chartoff, 2007), allowing us to access how brain reward function is modified by multiple cycles of nicotine exposure and withdrawal.
Animals underwent three treatment cycles; each cycle was composed of a chronic treatment period (nicotine or saline) followed by a withdrawal period (WD) (see Figure 1a). During chronic treatment over the course of all cycles, ICSS thresholds were not different between saline and nicotine groups. However, during the abstinence phase of withdrawal, reward thresholds were decreased in animals that underwent multiple cycles of chronic nicotine and withdrawal. Between-group analysis showed that chronic nicotine treatment was not different from saline on 1WD (F1,84=2.98, p=0.0989) and 2WD (F1,84=0.52, p=0.4799), but there was a significant effect of chronic nicotine treatment during 3WD (F1,52=6.13, p=0.0279) (Figure 1b).
Brain stimulation-reward thresholds and response latencies expressed as a percentage of pre-nicotine treatment values (mean values±SEM). Dashed line represents basal reward threshold set at 100% of baseline levels. (a) Experimental design. The segments depicted in black refer to the data presented bellow. (b) Asterisks indicate increased brain reward function in animals treated with nicotine minipump (NMP; 24 mg/kg/day) when compared with SMP animals, following multiple withdrawals, as seen during the third cycle of withdrawal (p<0.05). (c) Response latencies. Increase in the latency to respond during chronic treatment in the first cycle was similar in all animals but increased during withdrawal in NMP animals when compared with SMP (as indicated with the asterisk, p<0.05). Asterisks indicate higher latency to respond, in the second and third cycle, in NMP animals in both chronic treatment and withdrawal.
Response latency was increased in animals that experienced repeated chronic nicotine treatments and withdrawals (Figure 1c). A significant difference between saline and nicotine minipump groups was observed during the second (F1,84=7.42, p=0.0127) and third cycle of chronic treatment (F1,52=12.19, p=0.0040). Saline-treated animals showed lower latencies to respond during the first (F1,84=9.13, p=0.0065), second (F1,84=14.43, p=0.0011), and third (F1,52=18.03, p=0.001) periods of withdrawal compared with nicotine-treated mice.
ICSS Thresholds and Acute Nicotine Treatment
The effects of acute administration of nicotine in naive and nicotine-withdrawn mice vary in a number of behavioral tests. However, whether this extends to alterations of brain stimulation reward has not been examined. We found that 24 h following nicotine or SMP removal reward thresholds were actually increased following the first acute nicotine injection during 1WD, but returned to baseline with subsequent injections (see Figure 1a for experimental paradigm and Figure 2b). ANOVA comparing acute nicotine treatment days and baseline thresholds showed a significant difference between acute treatment days and baseline in SMP animals (F4,6=3.511, p=0.0216) and NMP animals (F4,8=2.86, p=0.0392) during 1WD. Newman–Keuls post hoc tests indicated a significant threshold elevation in SMP and NMP groups on day 1 compared with baseline (p<0.05), suggesting an aversive effect to the acute nicotine doses. A significant difference between day 1 and day 4 (p<0.05) was also observed in the SMP group, showing a decrease in anhedonia to the acute treatment by day 4. During 2WD and 3WD, no significant differences were observed between injection days and baseline in SMP animals (2WD: F4,6=0.2967, p=1.304; 3WD: F4,6=0.6346, p=0.6467). Interestingly, in NMP animals we observed a significant difference between acute treatment days and baseline (F4,8=6.836, p=0.0004). Further analysis with Newman–Keuls post hoc tests revealed a significant threshold decrease during day 4 when compared with baseline (p<0.05), indicative of hedonia. Moreover, significant decreases were found between day 4 and days 1 and 2 and between day 3 and day 1 (p<0.05), indicating an overall increase in the rewarding effect of nicotine with repeated injections. A significant difference between treatment days and baseline was also found in NMP animals during 3WD (F4,3=4.593, p=0.0176). Newman–Keuls post hoc tests indicated a significant threshold decrease during day 2 and 4 when compared with baseline (p<0.05). Thus, by the second and third cycle, the initial anhedonia associated with acute nicotine administration was no longer evident in SMP and NMP animals. Furthermore, the decreased thresholds observed in NMP animals during acute treatment showed an increased sensitivity to the rewarding effects of acute nicotine treatment with repeated withdrawals.
Brain stimulation-reward thresholds and response latencies expressed as a percentage of pre-nicotine treatment values (mean values±SEM). Dashed line represents basal reward threshold set at 100% of baseline levels. (a) Experimental design. The segments depicted in black refer to the data presented bellow. (b) Between-group comparisons. Asterisks indicate decreased reward sensitivity with acute nicotine treatment, during the first and second cycle of withdrawal in NMP and SMP groups when compared with NMP animals injected with saline (p<0.05), this effect was not observed during the third cycle of withdrawal. Asterisk during the third cycle of withdrawal indicates higher sensitivity to reward in both NMP groups, regardless of the injection administered, when compared with SMP groups (p<0.05). (Note: SMP and NMP symbols overlap on day 17). (c) Response latencies. Asterisks indicate lower latencies to respond in SMP animals when compared with NMP animals treated with nicotine or saline during first, second, or third cycle (p<0.05); moreover, nicotine injections reduced the time of response in NMP animals when compared with NMP animals injected with saline during third cycle (p<0.05).
Between-group analysis (ANOVA) revealed that nicotine injections had a significant effect on the thresholds during 1WD (F2,3=7.407, p=0.0240), 2WD (F2,3=15.72, p=0.0041), and 3WD (F2,3=35.00, p=0.0005). Newman–Keuls post hoc tests indicated significant elevations in ICSS thresholds, above baseline, in NMP and SMP mice injected with nicotine during 1WD and 2WD when compared with NMP mice injected with saline (p<0.05). However, by the third cycle (3WD), both NMP groups, regardless of acute saline or nicotine treatment, showed decreases in brain reward thresholds below baseline, while SMP animals injected with nicotine showed significantly higher thresholds compared with NMP animals (Newman–Keuls, p<0.05; Figure 2b), indicating that the effect of acute nicotine is more rewarding in animals that have undergone multiple withdrawals.
Response latency was significantly reduced by acute nicotine across all withdrawal periods (Figure 2c) (1WD: F2,60=4.91, p=0.0184; 2WD: F2,60=10.17, p=0.0009; 3WD: F2,36=39.56, p<0.0001). Further post hoc tests showed that SMP mice had faster response times than NMP mice in all WD periods (p<0.01). In addition, response latency was partially rescued as NMP animals, when injected with nicotine, had faster response times compared with saline injected NMP animals (p<0.01) (Figure 2c).
Reward Sensitivity and Withdrawal
While anhedonia was not observed following withdrawal from a dose of 24 mg/kg/day nicotine (Figure 1), animals exposed to a higher dose, 40 mg/kg/day for 14 days, had higher ICSS thresholds when compared with saline-treated animals (indicated by a main effect of treatment (F1,60=26.22, p<0.0001; Figure 3b). Further, Bonferroni post hoc analysis identified a significant difference between groups during days 1 and 2 of spontaneous withdrawal following MP removal (p<0.05) (Figure 3b). To further probe nicotine withdrawal behavior, the nicotinic receptor antagonist mecamylamine was administered on days 9, 11, and 13 of chronic nicotine/saline treatment. There was a main effect of drug (F2,24=6.48, p=0.0056) with significant elevations in ICSS thresholds occurring at 6 mg/kg of mecamylamine (Bonferroni post hoc, p<0.05; Figure 3c). The thresholds within injection administration were not significantly different from thresholds measured during nicotine treatment, before the acute treatments of mecamylamine.
(a) Experimental paradigm. (b) Effects of chronic administration of nicotine (40 mg/kg/day, 14 days) or saline and of spontaneous withdrawal on ICSS thresholds. The asterisk indicates a significant main effect of treatment during withdrawal (p<0.05). (c) Effect of mecamylamine-precipitated withdrawal. Brain reward thresholds are expressed as a percentage of the pretest day values. Asterisk indicates elevations in brain reward thresholds compared with those of the corresponding control group (p<0.05). Data are expressed as mean values±SEM.
Conditioned Place Preference
Key components of drug addiction are the compulsive drug-seeking and -taking behaviors that are triggered by maladaptive strong conditioning. CPP is routinely used to measure conditioned reward (Prus et al, 2009). We used CPP to evaluate the rewarding effects of nicotine in the acquisition and expression of a conditioned behavior and found that increasing the number of nicotine exposures and withdrawal periods enhanced nicotine-induced CPP. Nicotine had no effect on place preference in mice that were not previously exposed to nicotine, nor in mice that only had one chronic exposure to nicotine and were undergoing their first withdrawal. In contrast, significant place preference occurred after 2WD or 3WD in NMP exposed mice. A two-way ANOVA revealed conditioning day by treatment interaction (F(3,27)=7.72, p=0.0007, p<0.05, p<0.001; Bonferroni post hoc compared with saline). 3WD animals also showed a higher place preference relative to 1WD animals (p<0.01).
Receptor Up-Regulation
Long-term changes in brain reward circuitry by chronic nicotine may be related to persistent alterations in nAChR levels (Dani and Heinemann, 1996; Pidoplichko et al, 1997). Therefore, we investigated whether the progressive increase in reward sensitivity is associated with altered binding to nAChRs in animals experiencing multiple periods of nicotine exposure and withdrawal.
The brains of animals in the CPP experiments were used to measure nAChR levels with [3H]EB following 8 days of withdrawal. No up-regulation of receptors was observed in the cortex following multiple withdrawal periods (see Figure 4c). However, nAChRs were progressively increased in the striatum (F(3,23)=15.56, p<0.05) (see Figure 4d) across all withdrawal groups, and in the hippocampus at 3WD only (F(3,18)=5.804, p<0.05) (see Figure 4e).
(a) Experimental paradigm used for CPP, all groups receive only one set of four nicotine/saline injections administered in 4 consecutive days; 1WD group receives the injections during the first withdrawal, 2WD receives the injections during the second withdrawal, and 3WD group receives the injections during the third withdrawal. 2WD and 3WD groups have one and two withdrawal periods with no treatment, respectively. Saline group animals were treated with SMP and conditioned with nicotine acute injections. (b) Nicotine-induced place conditioning following conditioning with 0.5 mg/kg injection nicotine (s.c.). Animals preferred nicotine-paired environments only after 2 or 3 withdrawals (WD) compared with saline. 3WD animals also showed a higher place preference relative to 1WD animals (**p<0.01). (b–d) Effects of repeated withdrawals on nAChR modulation. (c) Cortical homogenates failed to show any significant up-regulation following multiple withdrawals. (d) Striatal homogenates revealed progressively higher density of nAChRs. (e) Hippocampal homogenates showed significantly higher levels of [3H]EB binding in samples from the 3WD group only. ***p<0.0001; **p<0.01; *p<0.05, compared with saline.
DISCUSSION
Tobacco use and abuse is a chronic, relapsing disorder in which compulsive drug-taking behavior persists despite negative consequences. The high addictive properties of nicotine are exemplified by the great difficulty individuals report in quitting smoking despite intrinsic and extrinsic motivation. While most attempts to quit smoking end in failure, there is a high level of interest in trying again, often within 30 days of the failed quit attempt (Fu et al, 2006). Therefore, understanding the underlying brain mechanisms associated with repeated nicotine exposure and withdrawal would be an important first step to promote tobacco dependence treatment among relapsed smokers. Using two complementary models, ICSS and CPP, we show that repeated cycles of nicotine exposure and withdrawal result in greater reward sensitivity to nicotine, particularly as the number of cycles increases.
Rewarding as well as aversive properties of nicotine may act synergistically to direct the behavior of smokers toward tobacco consumption (Koob, 1996). The magnitude of nicotine dependence, as well as the negative state and dysphoria associated with nicotine withdrawal, are good predictors of smoking relapse (Ockene et al, 2000). Indeed, ICSS studies in rats demonstrate reduced sensitivity to rewarding stimulation (anhedonia) following spontaneous withdrawal from nicotine minipump extraction (Skjei and Markou, 2003; Kenny et al, 2003a; Yamada et al, 2010; Epping-Jordan et al, 1998; Vlachou et al, 2011). However, the persistence of anhedonia following nicotine withdrawal is not a consistent finding in the literature. Self-administration studies in rats using ICSS to investigate changes in thresholds during withdrawal have reported: (a) unchanged brain reward thresholds (Paterson et al, 2007); (b) both elevations, during days 1–3 of withdrawal, and decreases, on extinction days 5–7 (Harris et al, 2011); and (c) long-term decreases in ICSS thresholds (Kenny and Markou, 2006b). Thus, negative motivation may not be the only factor underlying nicotine seeking and relapse. In mice, elevated ICSS thresholds (anhedonia) were observed during naturally induced withdrawal following 14 days of chronic nicotine (Johnson et al, 2008; Stoker et al, 2008). However, another study failed to observe the withdrawal-induced increase in thresholds with either 24 or 40 mg/kg/day for 14 days, but attained a significant elevation only by extending the 40 mg/kg/day treatment to 28 days (Stoker et al, 2008). More recent studies demonstrate the need for very high doses of chronic nicotine treatment to induce withdrawal in mice (40 and 80 mg/kg/day) (Stoker et al, 2012). There is a valid reason for administering high nicotine doses in mice due to the rate of nicotine metabolism. The approximate half-life of nicotine in humans is 120 min, but only 60 min in rats and 7–10 min in mice (Matta et al, 2007). Thus, the nicotine dose regimen employed in mice needs to be approximately tenfold higher in order to achieve nicotine plasma levels comparable to those of the rat (Matta et al, 2007). However, despite the need for higher doses of nicotine in mice, the high nicotine concentrations used in some studies are beyond those seen in typical smokers (Stolerman, 2010).
In our study, we observed anhedonia only with high doses of nicotine during mecamylamine-precipitated and spontaneous nicotine withdrawal. We failed to observe any somatic signs of withdrawal, such as jumping, rearing, shakes, abdominal constrictions, chewing, facial tremor, and scratching, following spontaneous withdrawal from 24 or 40 mg/kg/day at 24 h after minipump extraction similar to previous studies (Stoker et al, 2008). However, we observed increased response latencies in nicotine-treated animals, which might suggest a negative effect of nicotine withdrawal. Interestingly, we also observed a decline in the latency to respond in the saline animals with extended training. This effect was observed previously and interpreted as a result of motor-learning (Owesson-White et al, 2008). Thus, it is possible that a motor-learning impairment might be implicated in the increased response latencies of nicotine-treated animals.
To further examine the development of reward sensitization to nicotine, we used an unbiased CPP model. While no conditioned reward is evident following a single nicotine exposure and withdrawal period, multiple exposures and withdrawals significantly increased the positive response to acute nicotine. It is unlikely that this enhanced nicotine-induced place preference can be attributed to any residual nicotine due to the short half-life of nicotine in mice. Nicotine metabolites, such as cotinine, have a half-life range from 20 to 40 min in mice (Petersen et al, 1984), and are typically eliminated 24 h following nicotine administration. These data suggest that repeated withdrawal from nicotine is important in generating enhanced preference.
The development of nicotine tolerance, dependence, and withdrawal is contingent on nicotine-induced neuroadaptations, including desensitization and up-regulation of nAChRs (Quick and Lester, 2002). In our study, we hypothesized that repeated nicotine exposure and withdrawal contribute to a cholinergic system adaptation involving long-lasting up-regulation of receptors, specifically the α4β2* subtype, which is the most readily up-regulated subtype following chronic administration of nicotine (Gaimarri et al, 2007). This receptor-level adaptation to repeated nicotine exposure and withdrawal may underlie some of the behavioral phenomena we observe in mice. Similarly, imaging studies in humans proposed a functional role of these up-regulated nAChRs in relapse vulnerability (Cosgrove et al, 2009). Previously, nicotine receptor regulation was only determined after cessation of chronic nicotine treatment but not following repeated withdrawals (Collins et al, 1994; Hulihan-Giblin et al, 1990; Pietila et al, 1998; Turner et al, 2011). For example, in a previous time course study following a single 2-week administration of chronic nicotine, nicotinic receptors in the cortex and striatum were up-regulated for only 48 h and hippocampal nAChRs were up-regulated for only 24 h as measured by [3H]Epibatidine binding (Turner et al, 2011). We saw a significantly longer-lasting up-regulation of nAChRs, still present 8 days following nicotine cessation in both the striatum and hippocampus, after three nicotine exposures and withdrawals. This result is consistent with a recent SPECT study that showed nicotinic receptors remained elevated in smokers for up to 12 weeks (Cosgrove et al, 2009). Therefore, our data support the idea that multiple withdrawal periods might modulate the brain response to nicotine-induced cholinergic system imbalance by long-term alteration of receptor function.
Nicotine addiction involves neuroadaptations in the mesocorticolimbic dopamine system, which is paralleled by changes in nAChRs. The long-term up-regulation observed in our data in the striatum and hippocampus can be correlated with the roles these regions play in the addictive process. The striatum plays a crucial role in the development of habits and addiction (Koob and Volkow, 2010). Chronic nicotine is known to up-regulate α4β2* nAChRs in the striatum, thereby increasing dopamine release from dopaminergic terminals (Changeux, 2010). Nicotine's action on these receptors, and α6 receptors, causes a shift in the firing patterns of dopaminergic neurons from tonic, low frequency single-spike firing, to phasic, longer high frequency firing (De Biasi and Dani, 2011). A disproportionate decrease in the tonic firing compared with the phasic signals is also observed in the nucleus accumbens during withdrawal from chronic nicotine (Zhang et al, 2012). Chronic nicotine is also known to up-regulate α4β2* nAChRs in the hippocampus, inducing long-term potentiation (Changeux, 2010). The hippocampus is required for the association between drug reward and environmental cues (Packard and McGaugh, 1996). α4*, β2*, and α6* receptors are important for the acquisition of CPP (Changeux, 2010), a behavior that relies on the hippocampal associative function (Packard and McGaugh, 1996). Thus, it is possible that the up-regulation of these receptors contributes to the increased contextual relevance of the nicotine-paired compartment, observed in the current study.
Employing a paradigm of nicotine administration that incorporates cycles of withdrawal and nicotine exposure might not only induce long-term up-regulation of receptors, but also change the ratio of phasic relative to tonic firing of dopamine neurons. This phasic/tonic dopamine signaling ratio increase, as observed during withdrawal in previous studies (Grieder et al, 2012; Zhang et al, 2012), is implicated in habit learning (Aggarwal and Wickens, 2011), enhanced reward association and acquisition of incentive salience (Grieder et al, 2012). Thus, these studies might further explain the greater motivation to seek a reward, whether it is an electric stimulation or a substance of abuse, both observed here by the use of ICSS and CPP and the latter in a recent study by Cohen et al (2012), or the enhanced cue association, observed by Scott and Hiroi (2010), during withdrawal. Together, these studies support the incentive-motivational theory of withdrawal, where withdrawal enhances the incentive value of reward (Hutcheson et al, 2001), rendering withdrawal not as a rewarding state per se, but instead as a sensitized state to rewarding stimulation. The increased craving that characterizes human behavior following smoking cessation may in part be explained by enhanced hedonic processing that occurs following repeated withdrawal.
A key problem for the development of medications to treat nicotine addiction is the lack of animal models with sufficient predictive validity to support translation of pre-clinical findings to clinical research (Lerman et al, 2007). The promotion of therapies that target treatment among relapsed smokers interested in quitting is critical; however, to date few animal models have used paradigms that mimic the repeated cycle of nicotine exposure and withdrawal. Our results indicate that multiple nicotine withdrawal periods increase brain reward function, thereby potentiating the hedonic effects of nicotine. We suggest that this paradigm might prove to be useful as a closer approximation of smoking or tobacco use and as such an important animal model to evaluate mechanisms underlying nicotine addiction.
References
Aggarwal M, Wickens JR (2011). A role for phasic dopamine neuron firing in habit learning. Neuron 72: 892–894.
Carlezon Jr WA, Chartoff EH (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2: 2987–2995.
Changeux JP (2010). Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11: 389–401.
Cohen A, Koob GF, George O (2012). Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology; e-pub ahead of print 2 May 2012; doi:10.1038/npp.2012.67.
Collins AC, Luo Y, Selvaag S, Marks MJ (1994). Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther 271: 125–133.
Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T et al (2009). beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 66: 666–676.
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003a). Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 168: 347–358.
Cryan JF, Hoyer D, Markou A (2003b). Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54: 49–58.
Dani JA, Heinemann S (1996). Molecular and cellular aspects of nicotine abuse. Neuron 16: 905–908.
De Biasi M, Dani JA (2011). Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34: 105–130.
Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998). Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393: 76–79.
Fu SS, Partin MR, Snyder A, An LC, Nelson DB, Clothier B et al (2006). Promoting repeat tobacco dependence treatment: are relapsed smokers interested? Am J Manag Care 12: 235–243.
Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C (2007). Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev 55: 134–143.
Grabus SD, Martin BR, Brown SE, Damaj MI (2006). Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 184: 456–463.
Grieder TE, George O, Tan H, George SR, Le Foll B, Laviolette SR et al (2012). Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc Natl Acad Sci USA 109: 3101–3106.
Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG (2011). A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 217: 153–166.
Harrison AA, Gasparini F, Markou A (2002). Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160: 56–66.
Ho N, Balu DT, Hilario MR, Blendy JA, Lucki I (2012). Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiol Behav 105: 702–708.
Hulihan-Giblin BA, Lumpkin MD, Kellar KJ (1990). Effects of chronic administration of nicotine on prolactin release in the rat: inactivation of prolactin response by repeated injections of nicotine. J Pharmacol Exp Ther 252: 21–25.
Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A (2001). The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4: 943–947.
Johnson PM, Hollander JA, Kenny PJ (2008). Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol Biochem Behav 90: 409–415.
Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006a). Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26: 5894–5900.
Kenny PJ, Gasparini F, Markou A (2003a). Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther 306: 1068–1076.
Kenny PJ, Markou A (2006b). Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31: 1203–1211.
Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F et al (2003b). Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann NY Acad Sci 1003: 415–418.
Koob GF (1996). Drug addiction: the yin and yang of hedonic homeostasis. Neuron 16: 893–896.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Krall EA, Garvey AJ, Garcia RI (2002). Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 4: 95–100.
Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL et al (2007). Translational research in medication development for nicotine dependence. Nat Rev Drug Discov 6: 746–762.
Markou A, Koob GF (1992). Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51: 111–119.
Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR et al (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190: 269–319.
Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC et al (2000). Relapse and maintenance issues for smoking cessation. Health Psychol 19 (1 Suppl): 17–31.
Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM (2008). Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci USA 105: 11957–11962.
Packard MG, McGaugh JL (1996). Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65: 65–72.
Papke RL, Sanberg PR, Shytle RD (2001). Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther 297: 646–656.
Paterson NE, Balfour DJ, Markou A (2007). Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci 25: 3099–3108.
Paterson NE, Myers C, Markou A (2000). Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 152: 440–446.
Paxinos GF, Franklin K (2003). The Mouse Brain in Stereotaxic Coordinates, 3rd edn. Elsevier Academic Press: San Diego, CA.
Petersen DR, Norris KJ, Thompson JA (1984). A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos 12: 725–731.
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390: 401–404.
Pietila K, Lahde T, Attila M, Ahtee L, Nordberg A (1998). Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedebergs Arch Pharmacol 357: 176–182.
Prus AJ, James JR, Rosecrans JA (2009). Conditioned place preference. In: Buccafusco JJ (ed). Methods of Behavior Analysis in Neuroscience, 2nd edn. CRC Press: Boca Raton (FL). Ch. 4.
Quick MW, Lester RA (2002). Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478.
Risinger FO, Oakes RA (1995). Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav 51: 457–461.
Scott D, Hiroi N (2010). Emergence of dormant conditioned incentive approach by conditioned withdrawal in nicotine addiction. Biol Psychiatry 68: 726–732.
Skjei KL, Markou A (2003). Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 168: 280–292.
Stoker AK, Olivier B, Markou A (2012). Role of alpha7- and beta4-containing nicotinic acetylcholine receptors in the affective and somatic aspects of nicotine withdrawal: studies in knockout mice. Behav Genet 42: 423–436.
Stoker AK, Semenova S, Markou A (2008). Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54: 1223–1232.
Stolerman IP (2010). Encyclopedia of Psychopharmacology, Vol 2, 1st edn. Springer: New York.
Turner JR, Castellano LM, Blendy JA (2011). Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13: 41–46.
Vlachou S, Paterson NE, Guery S, Kaupmann K, Froestl W, Banerjee D et al (2011). Both GABA(B) receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats. Eur J Pharmacol 655: 52–58.
Yamada H, Bishnoi M, Keijzers KF, van Tuijl IA, Small E, Shah HP et al (2010). Preadolescent tobacco smoke exposure leads to acute nicotine dependence but does not affect the rewarding effects of nicotine or nicotine withdrawal in adulthood in rats. Pharmacol Biochem Behav 95: 401–409.
Zhang L, Dong Y, Doyon WM, Dani JA (2012). Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry 71: 184–191.
Acknowledgements
We thank Stephen Mague and Luis Tuesta for support with ICSS protocols. This research was supported by Grants T32-DA028874 (MRFH) and P50-CA-02585-01 (JAB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hilario, M., Turner, J. & Blendy, J. Reward Sensitization: Effects of Repeated Nicotine Exposure and Withdrawal in Mice. Neuropsychopharmacol 37, 2661–2670 (2012). https://doi.org/10.1038/npp.2012.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.130
Keywords
This article is cited by
-
Effect of menthol on nicotine intake and relapse vulnerability in a rat model of concurrent intravenous menthol/nicotine self-administration
Psychopharmacology (2019)
-
12-h abstinence-induced functional connectivity density changes and craving in young smokers: a resting-state study
Brain Imaging and Behavior (2019)
-
Age- and Nicotine-Associated Gene Expression Changes in the Hippocampus of APP/PS1 Mice
Journal of Molecular Neuroscience (2019)
-
Chronic nicotine differentially affects murine transcriptome profiling in isolated cortical interneurons and pyramidal neurons
BMC Genomics (2017)
-
Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice
Psychopharmacology (2015)