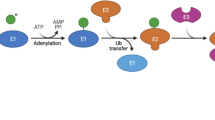
Key Points
-
When a cell is confronted by stress, p53 is stabilized in the nucleus, where it initiates cellular responses through transcriptional activation or repression of distinct target genes that primarily function to prevent proliferation of damaged cells.
-
The function of p53 is tightly controlled by its interaction with negative regulators including MDM2, which induces p53 degradation and prevents its accumulation in normal cells. This interaction can be disrupted when the cell detects DNA damage or other stresses, resulting in stabilization and activation of p53.
-
Active p53 is subject to a diverse array of covalent post-translational modifications, which markedly influence the expression of p53 target genes.
-
Phosphorylation and acetylation of p53 generally result in its stabilization and accumulation in the nucleus, followed by activation. Significant redundancies are observed in that the same p53 site is phosphorylated by several different protein kinases and distinct protein kinases also phosphorylate several sites on p53.
-
Mutant p53 proteins generally show intense phosphorylation and acetylation at sites that are well known to stabilize wild-type p53, and so could facilitate accumulation of dysfunctional mutant p53 in the nucleus, where it can act as an oncogene.
-
Overexpression of MDM2 E3 ubiquitin ligase is observed in many tumour types and results in the aberrant deactivation of p53.
-
In normal cells, p53 post-translational modification is induced by numerous carcinogens. Evidence indicates that normal cells and cancer cells show a markedly different response to ultraviolet-light exposure.
-
Dietary-derived chemopreventive agents induce phosphorylation of p53, resulting in cell-cycle arrest or apoptosis. These agents might have a preventive function in future anticancer therapies.
Abstract
Interest in the tumour suppressor p53 has generated much information regarding the complexity of its function and regulation in carcinogenesis. However, gaps still exist in our knowledge regarding the role of p53 post-translational modifications in carcinogenesis and cancer prevention. A thorough understanding of p53 will be extremely useful in the development of new strategies for treating and preventing cancer, including restoration of p53 function and selective killing of tumours with mutant TP53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Friedman, P. N., Chen, X., Bargonetti, J. & Prives, C. The p53 protein is an unusually shaped tetramer that binds directly to DNA. Proc. Natl Acad. Sci. USA 90, 3319–3323 (1993).
Davison, T. S., Yin, P., Nie, E., Kay, C. & Arrowsmith, C. H. Characterization of the oligomerization defects of two p53 mutants found in families with Li–Fraumeni and Li–Fraumeni-like syndrome. Oncogene 17, 651–656 (1998).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Olivier, M., Hussain, S. P., Caron de Fromentel, C., Hainaut, P. & Harris, C. C. TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. IARC Sci. Publ. 157, 247–270 (2004).
Craven, R. J., Lightfoot, H. & Cance, W. G. A decade of tyrosine kinases: from gene discovery to therapeutics. Surg. Oncol. 12, 39–49 (2003).
Shaw, P., Freeman, J., Bovey, R. & Iggo, R. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 12, 921–930 (1996).
Wesierska-Gadek, J., Bugajska-Schretter, A. & Cerni, C. ADP-ribosylation of p53 tumor suppressor protein: mutant but not wild-type p53 is modified. J. Cell Biochem. 62, 90–101 (1996).
Ito, A. et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20, 1331–1340 (2001).
Espinosa, J. M. & Emerson, B. M. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol. Cell 8, 57–69 (2001). Questions the importance of acetylation of p53.
Nakamura, S., Roth, J. A. & Mukhopadhyay, T. Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination. Mol. Cell Biol. 20, 9391–9398 (2000).
Barlev, N. A. et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8, 1243–1254 (2001).
Luo, J., Su, F., Chen, D., Shiloh, A. & Gu, W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408, 377–381 (2000).
Juan, L. J. et al. Histone deacetylases specifically down-regulate p53-dependent gene activation. J. Biol. Chem. 275, 20436–20443 (2000).
Vaziri, H. et al. SIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Rodriguez, M. S., Desterro, J. M., Lain, S., Lane, D. P. & Hay, R. T. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol. Cell Biol. 20, 8458–8467 (2000).
Xirodimas, D. P., Saville, M. K., Bourdon, J. C., Hay, R. T. & Lane, D. P. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118, 83–97 (2004). The first paper to report neddylation as a post-translational modification of p53.
Hupp, T. R. & Lane, D. P. Allosteric activation of latent p53 tetramers. Curr. Biol. 4, 865–875 (1994).
Gatti, A., Li, H. H., Traugh, J. A. & Liu, X. Phosphorylation of human p53 on Thr-55. Biochemistry 39, 9837–9842 (2000).
Waterman, M. J., Stavridi, E. S., Waterman, J. L. & Halazonetis, T. D. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nature Genet. 19, 175–178 (1998).
Sakaguchi, K. et al. Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. Effect on Mdm2 binding. J. Biol. Chem. 275, 9278–9283 (2000).
Dumaz, N., Milne, D. M. & Meek, D. W. Protein kinase CK1 is a p53-threonine 18 kinase which requires prior phosphorylation of serine 15. FEBS Lett. 463, 312–316 (1999).
Buschmann, T. et al. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol. Cell. Biol. 21, 2743–2754 (2001).
Haneda, M. et al. Protein phosphatase 1, but not protein phosphatase 2A, dephosphorylates DNA-damaging stress-induced phospho-serine 15 of p53. FEBS Lett. 567, 171–174 (2004).
Fan, G., Ma, X., Wong, P. Y., Rodrigues, C. M. & Steer, C. J. p53 dephosphorylation and p21(Cip1/Waf1) translocation correlate with caspase-3 activation in TGF-β1-induced apoptosis of HuH-7 cells. Apoptosis 9, 211–221 (2004).
Buschmann, T., Adler, V., Matusevich, E., Fuchs, S. Y. & Ronai, Z. p53 phosphorylation and association with murine double minute 2, c-Jun NH2-terminal kinase, p14ARF, and p300/CBP during the cell cycle and after exposure to ultraviolet irradiation. Cancer Res. 60, 896–900 (2000).
Ullrich, S. J. et al. Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumors is altered compared to wild-type p53. Proc. Natl Acad. Sci. USA 90, 5954–5958 (1993). One of the first reports to show that post-translational modification of mutant p53 differs from wild-type p53.
Higashimoto, Y. et al. Human p53 is phosphorylated on serines 6 and 9 in response to DNA damage-inducing agents. J. Biol. Chem. 275, 23199–23203 (2000).
Kao, C. F., Chen, S. Y., Chen, J. Y. & Wu Lee, Y. H. Modulation of p53 transcription regulatory activity and post–translational modification by hepatitis C virus core protein. Oncogene 23, 2472–2483 (2004).
Ray, R. B. & Ray, R. Hepatitis C virus core protein: intriguing properties and functional relevance. FEMS Microbiol. Lett. 202, 149–156 (2001).
Frazier, M. W. et al. Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain. Mol. Cell. Biol. 18, 3735–3743 (1998).
Atema, A. & Chene, P. The gain of function of the p53 mutant Asp281Gly is dependent on its ability to form tetramers. Cancer Lett. 185, 103–109 (2002).
Chene, P. In vitro analysis of the dominant negative effect of p53 mutants. J. Mol. Biol. 281, 205–209 (1998).
Chan, W. M., Siu, W. Y., Lau, A. & Poon, R. Y. How many mutant p53 molecules are needed to inactivate a tetramer? Mol. Cell. Biol. 24, 3536–3551 (2004).
Nicholls, C. D., McLure, K. G., Shields, M. A. & Lee, P. W. Biogenesis of p53 involves cotranslational dimerization of monomers and posttranslational dimerization of dimers. Implications on the dominant negative effect. J. Biol. Chem. 277, 12937–12945 (2002).
Willis, A., Jung, E. J., Wakefield, T. & Chen, X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene 23, 2330–2338 (2004).
Minamoto, T. et al. Distinct pattern of p53 phosphorylation in human tumors. Oncogene 20, 3341–3347 (2001). One of only a few studies that examined the phosphorylation pattern in a range of cancer cell types that express mutant p53.
Melnikova, V. O., Santamaria, A. B., Bolshakov, S. V. & Ananthaswamy, H. N. Mutant p53 is constitutively phosphorylated at Serine 15 in UV-induced mouse skin tumors: involvement of ERK1/2 MAP kinase. Oncogene 22, 5958–5966 (2003).
Sakaguchi, K. et al. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry 36, 10117–10124 (1997).
Furihata, M. et al. Frequent phosphorylation at serine 392 in overexpressed p53 protein due to missense mutation in carcinoma of the urinary tract. J. Pathol. 197, 82–88 (2002).
Yap, D. B. et al. Ser392 phosphorylation regulates the oncogenic function of mutant p53. Cancer Res. 64, 4749–4754 (2004).
Zhong, S., Salomoni, P. & Pandolfi, P. P. The transcriptional role of PML and the nuclear body. Nature Cell Biol. 2, E85–E90 (2000).
Guo, A. et al. The function of PML in p53-dependent apoptosis. Nature Cell Biol. 2, 730–736 (2000).
Borden, K. L. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell. Biol. 22, 5259–5269 (2002).
Negorev, D. & Maul, G. G. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 20, 7234–7242 (2001).
Muratani, M. et al. Metabolic-energy-dependent movement of PML bodies within the mammalian cell nucleus. Nature Cell Biol. 4, 106–110 (2002).
Pearson, M. et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207–210 (2000). One of the first studies to report that PML NBs are required for RAS-induced p53 acetylation (at Lys382) by the CBP acetyltransferase.
Langley, E. et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 21, 2383–2396 (2002).
Louria-Hayon, I. et al. The promyelocytic leukemia protein protects p53 from Mdm2-mediated inhibition and degradation. J. Biol. Chem. 278, 33134–33141 (2003).
Insinga, A. et al. Impairment of p53 acetylation, stability and function by an oncogenic transcription factor. EMBO J. 23, 1144–1154 (2004).
Ishov, A. M. et al. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147, 221–234 (1999).
Rodriguez, M. S. et al. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18, 6455–6461 (1999).
Gostissa, M. et al. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 18, 6462–6471 (1999).
Melchior, F. & Hengst, L. SUMO-1 and p53. Cell Cycle 1, 245–249 (2002).
Chen, L. & Chen, J. MDM2–ARF complex regulates p53 sumoylation. Oncogene 22, 5348–5357 (2003).
Xirodimas, D. P., Chisholm, J., Desterro, J. M., Lane, D. P. & Hay, R. T. P14ARF promotes accumulation of SUMO-1 conjugated (H)Mdm2. FEBS Lett. 528, 207–211 (2002).
Girdwood, D. et al. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11, 1043–1054 (2003).
Kim, Y. H., Choi, C. Y. & Kim, Y. Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc. Natl Acad. Sci. USA 96, 12350–12355 (1999).
Fuchs, S. Y., Adler, V., Buschmann, T., Wu, X. & Ronai, Z. Mdm2 association with p53 targets its ubiquitination. Oncogene 17, 2543–2547 (1998).
Honda, R., Tanaka, H. & Yasuda, H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 (1997).
Blattner, C., Hay, T., Meek, D. W. & Lane, D. P. Hypophosphorylation of Mdm2 augments p53 stability. Mol. Cell. Biol. 22, 6170–6182 (2002).
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003). Indicates that the level of MDM2 activity determines whether p53 will be exported or degraded in the nucleus.
Knights, C. D., Liu, Y., Appella, E. & Kulesz-Martin, M. Defective p53 post-translational modification required for wild type p53 inactivation in malignant epithelial cells with mdm2 gene amplification. J. Biol. Chem. 278, 52890–52900 (2003).
Lavin, M. F. & Shiloh, Y. The genetic defect in ataxia-telangiectasia. Annu. Rev. Immunol. 15, 177–202 (1997).
Appella, E. & Anderson, C. W. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268, 2764–2772 (2001).
Xia, L., Paik, A. & Li, J. J. p53 activation in chronic radiation-treated breast cancer cells: regulation of MDM2/p14ARF. Cancer Res. 64, 221–228 (2004).
O'Leary, K. A., Mendrysa, S. M., Vaccaro, A. & Perry, M. E. Mdm2 regulates p53 independently of p19ARF in homeostatic tissues. Mol. Cell. Biol. 24, 186–191 (2004). Indicates that different p53 regulatory pathways are activated in normal and stressed cells.
Finch, R. A. et al. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62, 3221–3225 (2002).
Migliorini, D. et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 22, 5527–5538 (2002).
Ramos, Y. F. et al. Aberrant expression of HDMX proteins in tumor cells correlates with wild-type p53. Cancer Res. 61, 1839–1842 (2001).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002). The first paper to identify HAUSP as a protein that functions to de-ubiquitylate p53.
Lim, S. K., Shin, J. M., Kim, Y. S. & Baek, K. H. Identification and characterization of murine mHAUSP encoding a deubiquitinating enzyme that regulates the status of p53 ubiquitination. Int. J. Oncol. 24, 357–364 (2004).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53–Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Pitot, H. C. & Dragan, Y. P. The multistage nature of chemically induced hepatocarcinogenesis in the rat. Drug Metab. Rev. 26, 209–220 (1994).
Lee, Y. I., Lee, S., Das, G. C., Park, U. S. & Park, S. M. Activation of the insulin-like growth factor II transcription by aflatoxin B1 induced p53 mutant 249 is caused by activation of transcription complexes; implications for a gain-of-function during the formation of hepatocellular carcinoma. Oncogene 19, 3717–3726 (2000).
Chaturvedi, V., Qin, J. Z., Stennett, L., Choubey, D. & Nickoloff, B. J. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J. Cell Physiol. 198, 100–109 (2004).
Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677 (1998).
Canman, C. E. et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677–1679 (1998).
Khanna, K. K. et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nature Genet. 20, 398–400 (1998).
Zhang, Y., Ma, W. Y., Kaji, A., Bode, A. M. & Dong, Z. Requirement of ATM in UVA-induced signaling and apoptosis. J. Biol. Chem. 277, 3124–3131 (2002). The first study to show that ATM is activated differentially in response to various wavelengths of ultraviolet irradiation.
Unsal-Kacmaz, K., Makhov, A. M., Griffith, J. D. & Sancar, A. Preferential binding of ATR protein to UV-damaged DNA. Proc. Natl Acad. Sci. USA 99, 6673–6678 (2002).
Hong, W. K. General keynote: the impact of cancer chemoprevention. Gynecol. Oncol. 88, S56–S58 (2003).
Forbes, I. J., Zalewski, P. D., Giannakis, C. & Cowled, P. A. Induction of apoptosis in chronic lymphocytic leukemia cells and its prevention by phorbol ester. Exp. Cell Res. 198, 367–372 (1992).
Song, Q., Baxter, G. D., Kovacs, E. M., Findik, D. & Lavin, M. F. Inhibition of apoptosis in human tumour cells by okadaic acid. J. Cell Physiol. 153, 550–556 (1992).
Tomei, L. D., Kanter, P. & Wenner, C. E. Inhibition of radiation-induced apoptosis in vitro by tumor promoters. Biochem. Biophys. Res. Commun. 155, 324–331 (1988).
Dercks, W. & Creasy, L. L. The significance of stilbene phytoalexins in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Path. 34, 189–202 (1989).
Kim, Y. A. et al. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 11, 441–446 (2004).
Shih, A., Davis, F. B., Lin, H. Y. & Davis, P. J. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J. Clin. Endocrinol. Metab. 87, 1223–1232 (2002).
She, Q. B. et al. Inhibition of cell transformation by resveratrol and its derivatives: differential effects and mechanisms involved. Oncogene 22, 2143–2150 (2003).
She, Q. B., Bode, A. M., Ma, W. Y., Chen, N. Y. & Dong, Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61, 1604–1610 (2001).
Huang, C., Ma, W. Y., Goranson, A. & Dong, Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis 20, 237–242 (1999).
Lu, J., Ho, C. T., Ghai, G. & Chen, K. Y. Differential effects of theaflavin monogallates on cell growth, apoptosis, and Cox-2 gene expression in cancerous versus normal cells. Cancer Res. 60, 6465–6471 (2000).
Hastak, K. et al. Role of p53 and NF-κB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene 22, 4851–4859 (2003).
Kuo, P. L. & Lin, C. C. Green tea constituent (–)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J. Biomed. Sci. 10, 219–227 (2003).
Sah, J. F., Balasubramanian, S., Eckert, R. L. & Rorke, E. A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 279, 12755–12762 (2004).
He, Z. et al. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 63, 4396–4401 (2003).
Hashimoto, T. et al. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 64, 3344–3349 (2004).
Sarkaria, J. N. et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 (1999).
Cortez, D. Caffeine inhibits checkpoint responses without inhibiting the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases. J. Biol. Chem. 278, 37139–37145 (2003).
Chen, J., Lin, J. & Levine, A. J. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol. Med. 1, 142–152 (1995).
Stommel, J. M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660–1672 (1999).
Liu, L. et al. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19, 1202–1209 (1999).
Lill, N. L., Grossman, S. R., Ginsberg, D., DeCaprio, J. & Livingston, D. M. Binding and modulation of p53 by p300/CBP coactivators. Nature 387, 823–827 (1997).
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997).
Avantaggiati, M. L. et al. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89, 1175–1184 (1997).
Luo, J. et al. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl Acad. Sci. USA 101, 2259–2264 (2004).
Wang, Y. H., Tsay, Y. G., Tan, B. C., Lo, W. Y. & Lee, S. C. Identification and characterization of a novel p300-mediated p53 acetylation site, lysine 305. J. Biol. Chem. 278, 25568–25576 (2003).
Wang, X., Taplick, J., Geva, N. & Oren, M. Inhibition of p53 degradation by Mdm2 acetylation. FEBS Lett. 561, 195–201 (2004).
Jin, Y., Zeng, S. X., Lee, H. & Lu, H. MDM2 mediates p300/CREB-binding protein-associated factor ubiquitination and degradation. J. Biol. Chem. 279, 20035–20043 (2004).
Smith, J. S. et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl Acad. Sci. USA 97, 6658–6663 (2000).
Florenes, V. A., Skrede, M., Jorgensen, K. & Nesland, J. M. Deacetylase inhibition in malignant melanomas: impact on cell cycle regulation and survival. Melanoma Res. 14, 173–181 (2004).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002).
Ferbeyre, G. et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14, 2015–2027 (2000).
Bischof, O. et al. Deconstructing PML-induced premature senescence. EMBO J. 21, 3358–3369 (2002).
de Stanchina, E. et al. PML is a direct p53 target that modulates p53 effector functions. Mol. Cell 13, 523–535 (2004).
Fuchs, S. Y. et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 12, 2658–2663 (1998). One of the first reports illustrating a function for JNK in the regulation of p53.
Fuchs, S. Y., Adler, V., Pincus, M. R. & Ronai, Z. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl Acad. Sci. USA 95, 10541–10546 (1998).
Scheffner, M., Huibregtse, J. M., Vierstra, R. D. & Howley, P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495–505 (1993).
Leng, R. P. et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112, 779–791 (2003).
Dornan, D. et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429, 86–92 (2004).
Bech-Otschir, D. et al. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 20, 1630–1639 (2001).
Li, H. H., Li, A. G., Sheppard, H. M. & Liu, X. Phosphorylation on Thr-55 by TAF1 mediates degradation of p53: a role for TAF1 in cell G1 progression. Mol. Cell 13, 867–878 (2004).
Katayama, H. et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature Genet. 36, 55–62 (2004).
Pohler, E. et al. The Barrett's antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol. Cell Proteomics 3, 534–547 (2004).
Ohtsuka, T., Jensen, M. R., Kim, H. G., Kim, K. T. & Lee, S. W. The negative role of cyclin G in ATM-dependent p53 activation. Oncogene 23, 5405–5408 (2004).
Reimer, C. L. et al. Altered regulation of cyclin G in human breast cancer and its specific localization at replication foci in response to DNA damage in p53+/+ cells. J. Biol. Chem. 274, 11022–11029 (1999).
Derenzini, M. The AgNORs. Micron 31, 117–120 (2000).
Chan, W. Y. et al. Characterization of the cDNA encoding human nucleophosmin and studies of its role in normal and abnormal growth. Biochemistry 28, 1033–1039 (1989).
Maiguel, D. A., Jones, L., Chakravarty, D., Yang, C. & Carrier, F. Nucleophosmin sets a threshold for p53 response to UV radiation. Mol. Cell. Biol. 24, 3703–3711 (2004).
Saito, S. et al. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J. Biol. Chem. 277, 12491–12494 (2002).
Tibbetts, R. S. et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13, 152–157 (1999).
Blaydes, J. P. et al. Stoichiometric phosphorylation of human p53 at Ser315 stimulates p53-dependent transcription. J. Biol. Chem. 276, 4699–4708 (2001).
Chehab, N. H., Malikzay, A., Stavridi, E. S. & Halazonetis, T. D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc. Natl Acad. Sci. USA 96, 13777–13782 (1999).
Unger, T. et al. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 18, 1805–1814 (1999).
Knippschild, U. et al. p53 is phosphorylated in vitro and in vivo by the δ and ε isoforms of casein kinase 1 and enhances the level of casein kinase 1δ in response to topoisomerase-directed drugs. Oncogene 15, 1727–1736 (1997).
Shieh, S. Y., Ikeda, M., Taya, Y. & Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 (1997).
She, Q. B., Chen, N. & Dong, Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J. Biol. Chem. 275, 20444–20449 (2000).
Yeh, P. Y., Chuang, S. E., Yeh, K. H., Song, Y. C. & Cheng, A. L. Nuclear extracellular signal-regulated kinase 2 phosphorylates p53 at Thr55 in response to doxorubicin. Biochem. Biophys. Res. Commun. 284, 880–886 (2001).
Keller, D. M. et al. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol. Cell 7, 283–292 (2001).
Qu, L. et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 18, 261–277 (2004).
Hofmann, T. G. et al. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nature Cell Biol. 4, 1–10 (2002).
D'Orazi, G. et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nature Cell Biol. 4, 11–19 (2002).
She, Q. B., Ma, W. Y. & Dong, Z. Role of MAP kinases in UVB-induced phosphorylation of p53 at serine 20. Oncogene 21, 1580–1589 (2002).
Bulavin, D. V. et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18, 6845–6854 (1999).
Huang, C., Ma, W. Y., Maxiner, A., Sun, Y. & Dong, Z. p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem. 274, 12229–12235 (1999).
Chernov, M. V., Bean, L. J., Lerner, N. & Stark, G. R. Regulation of ubiquitination and degradation of p53 in unstressed cells through C-terminal phosphorylation. J. Biol. Chem. 276, 31819–31824 (2001).
Cuddihy, A. R., Wong, A. H., Tam, N. W., Li, S. & Koromilas, A. E. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18, 2690–2702 (1999).
Acknowledgements
The plethora of literature related to the response of p53 to stress makes a complete and extensive review extremely challenging and we apologize in advance for any inadvertent omission. This work is supported by the Hormel Foundation, grants from the National Institutes of Health and a grant from the American Institute for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Cancer.gov
Entrez Gene
FURTHER INFORMATION
International Agency for Research on Cancer TP53 Mutation Database
Glossary
- 26S PROTEASOME
-
The protease part of the ubiquitin system, which is the main proteolytic system in eukaryotic cells that degrades polyubiquitylated proteins.
- GLYCOSYLATION
-
The linkage of carbohydrates to protein either through the amide group of aspargine (N-glycosidic linkage) or through the hydoxyl of serine or threonine (O-glycosidic linkage).
- RIBOSYLATION
-
The attachment of poly(ADP-ribose) chains to proteins catalysed by the nuclear protein poly(ADP-ribosyl) transferase. Ribosylation contributes to the regulation of DNA repair and transcription. Ribosylation of transcription factors prevents their binding to DNA.
- NUCLEAR BODIES
-
Dynamic multiprotein complexes comprising numerous transient or permanently localized proteins located in the nucleus.
- MONOUBIQUITYLATION
-
Conjugation with a ubiquitin monomer at one or several lysines within the protein, which might regulate protein function and/or localization within the cell.
- POLYUBIQUITYLATION
-
Conjugation with a polymeric ubiquitin chain at one or more lysines within the protein that mark the protein for degradation through the 26S proteasome.
- AFLATOXIN
-
A toxin produced by some strains of Aspergillus flavus and A. parasiticus that has a carcinogenic effect in experimental animals; it can be present in peanuts or peanut products contaminated with Aspergillus moulds.
Rights and permissions
About this article
Cite this article
Bode, A., Dong, Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4, 793–805 (2004). https://doi.org/10.1038/nrc1455
Issue Date:
DOI: https://doi.org/10.1038/nrc1455
This article is cited by
-
Protein acetylation sites with complex-valued polynomial model
Frontiers of Computer Science (2024)
-
The emerging roles of SUMOylation in the tumor microenvironment and therapeutic implications
Experimental Hematology & Oncology (2023)
-
VprBP/DCAF1 regulates p53 function and stability through site-specific phosphorylation
Oncogene (2023)
-
Spatial transcriptomics analysis of esophageal squamous precancerous lesions and their progression to esophageal cancer
Nature Communications (2023)
-
Emerging posttranslational modifications and their roles in DNA damage response
Genome Instability & Disease (2023)