Key Points
-
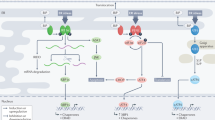
When eukaryotic cells encounter adverse physiological conditions that impact on protein folding in the endoplasmic reticulum, a signal-transduction cascade is activated; this is termed the unfolded protein response (UPR).
-
The UPR is a multipronged response that is largely cytoprotective, but under conditions of prolonged stress apoptotic components are activated.
-
The UPR can be activated in tumours; this is apparently due to their inadequate vascularization. This results in limited oxygen and nutrients, which impinge on the normal maturation of secretory-pathway proteins.
-
Activation of the UPR might promote dormancy, aid tumour growth or protect the host by inducing apoptosis. It is presently unclear where the balance lies and how the fate of a tumour cell is eventually decided, although the part of the UPR pathway that upregulates endoplasmic reticulum chaperones seems to have a protective role during tumour development.
-
In vitro activation of the UPR alters the sensitivity of tumour cells to chemotherapeutic agents.
-
Although several interesting observations have been made to implicate the UPR in tumour growth and resistance to treatments, more comprehensive in vivo studies need to be done to determine how large of a role it has and to identify the most important components of the pathway, and to determine which stages of tumour development are regulated by the UPR.
Abstract
Having accumulated mutations that overcome cell-cycle and apoptotic checkpoints, the main obstacle to survival faced by a cancer cell is the restricted supply of nutrients and oxygen. These conditions impinge on protein folding in the endoplasmic reticulum and activate a largely cytoprotective signalling pathway called the unfolded protein response. Prolonged activation of this response can, however, terminate in apoptosis. Recent delineation of the components of this response, coupled with several clinical studies, indicate that it is uniquely poised to have a role in regulating the balance between cancer cell death, dormancy and aggressive growth, as well as altering the sensitivity of solid tumours to chemotherapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lee, A. S. Mammalian stress response: induction of the glucose-regulated protein family. Curr. Opin. Cell Biol. 4, 267–273 (1992).
Ellgaard, L., Molinari, M. & Helenius, A. Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888 (1999).
Ma, Y. & Hendershot, L. M. ER chaperone functions during normal and stress conditions. J. Chem. Neuro. 28, 51–65 (2004).
Brodsky, J. L. et al. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J. Biol. Chem. 274, 3453–3460 (1999).
Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988). A range of pharmacological agents had been used to activate an undefined signal-transduction cascade that led to the coordinate upregulation of resident ER chaperones. The authors of this paper hypothesized that all of the agents used could affect normal protein folding in the ER and demonstrated that the upstream signal was the presence of unfolded proteins in the ER.
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signalling from the ER to the nucleus. Cell 74, 743–756 (1993).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Okamura, K., Kimata, Y., Higashio, H., Tsuru, A. & Kohno, K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 279, 445–450 (2000).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 (1997). Demonstrated that during ER stress the endonuclease domain of the Ire1 kinase is activated to cleave the mRNA of the Hac1 transcription factor, allowing it to be synthesized and transactivate target genes of the UPR. This laid the foundation for future efforts to decipher the UPR pathway in higher organisms.
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Imaizumi, K. et al. The unfolded protein response and Alzheimer's disease. Biochim. Biophys. Acta 1536, 85–96 (2001).
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J. & Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 (2003).
Pelled, D. et al. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J. Biol. Chem. 278, 29496–29501 (2003).
Tessitore, A. et al. G(M1)-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol. Cell 15, 753–766 (2004).
Feng, B. et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 5, 781–792 (2003).
Lee, A. S. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem. Sci. 26, 504–510 (2001).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
Iwakoshi, N. N. et al. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature Immunol. 4, 321–329 (2003).
Reimold, A. M. et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 14, 152–157 (2000).
Gass, J. N., Gifford, N. M. & Brewer, J. W. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem. 277, 49047–49054 (2002).
Fernandez, P. M. et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res. Treat. 59, 15–26 (2000). Correlated increased malignancy of human breast cancers malignancy to upregulation of BiP at both the transcriptional and translational level.
Shuda, M. et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 38, 605–614 (2003). Demonstrated the activation of both the ATF6 and IRE1-dependent branches of the UPR pathway in human hepatocellular carcinoma tissue samples at both the mRNA and protein level.
Song, M. S., Park, Y. K., Lee, J. H. & Park, K. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-ε/ERK/AP-1 signaling cascade. Cancer Res. 61, 8322–8330 (2001).
Chen, X., Ding, Y., Liu, C. G., Mikhail, S. & Yang, C. S. Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis 23, 123–130 (2002).
Gazit, G., Lu, J. & Lee, A. S. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res. Treat. 54, 135–146 (1999).
Shen, J. et al. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc. Natl Acad. Sci. USA 84, 3278–3282 (1987).
Ozawa, K. et al. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 61, 4206–4213 (2001). Showed that GRP170 , a gene that is regulated by both hypoxic and ER-stress response pathways, has an important role in facilitating the secretion of VEGF in vitro and angiogenesis during tumour development in vivo.
Jamora, C., Dennert, G. & Lee, A. S. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA 93, 7690–7694 (1996). Directly demonstrated the significance of ER-chaperone upregulation in tumour progression.
Park, H. R. et al. Effect on tumor cells of blocking survival response to glucose deprivation. J. Natl Cancer Inst. 96, 1300–1310 (2004).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Lee, A. H., Iwakoshi, N. N., Anderson, K. C. & Glimcher, L. H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003).
Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 (2004).
Rajendran, J. G. et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin. Cancer Res. 10, 2245–2252 (2004).
Sivridis, E., Giatromanolaki, A. & Koukourakis, M. I. The vascular network of tumours: what is it not for? J. Pathol. 201, 173–180 (2003).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000). Identified a new section of the UPR that is found in higher eukaryotes. This signalling pathway is downstream of PERK and activates a distinct group of genes that are also activated by various other cellular stress conditions, including amino-acid deprivation and hypoxia.
Ma, Y. & Hendershot, L. M. Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J. Biol. Chem. 279, 13792–13799 (2004).
Poellinger, L. & Johnson, R. S. HIF-1 and hypoxic response: the plot thickens. Curr. Opin. Genet. Dev. 14, 81–85 (2004).
Semenza, G. L. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107, 1–3 (2001).
Pugh, C. W. & Ratcliffe, P. J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nature Med. 9, 677–684 (2003).
Koumenis, C. et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol. Cell. Biol. 22, 7405–7416 (2002). Demonstrated an overlap between one section of the hypoxic response and the UPR.
Blais, J. D. et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol. Cell. Biol. 24, 7469–7482 (2004).
Koong, A. C., Chen, E. Y. & Giaccia, A. J. Hypoxia causes the activation of nuclear factor κB through the phosphorylation of IκBα on tyrosine residues. Cancer Res. 54, 1425–1430 (1994).
Jiang, H. Y. et al. Phosphorylation of the α subunit of eukaryotic initiation factor 2 is required for activation of NF-κB in response to diverse cellular stresses. Mol. Cell. Biol. 23, 5651–5663 (2003).
Alarcon, R., Koumenis, C., Geyer, R. K., Maki, C. G. & Giaccia, A. J. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 59, 6046–6051 (1999).
Qu, L. et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 18, 261–277 (2004). Showed that p53 is regulated by both the hypoxic and the ER-stress response pathways, but in opposite ways.
Ferrara, N. & Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 18, 4–25 (1997).
Roybal, C. N. et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J. Biol. Chem. 279, 14844–14852 (2004).
Ikeda, J. et al. Cloning and expression of cDNA encoding the human 150 kDa oxygen-regulated protein, ORP150. Biochem. Biophys. Res. Commun. 230, 94–99 (1997).
Ozawa, K. et al. Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport. J. Clin. Invest. 108, 41–50 (2001).
Steel, G. J., Fullerton, D. M., Tyson, J. R. & Stirling, C. J. Coordinated activation of Hsp70 chaperones. Science 303, 98–101 (2004).
Nielsen, M., Thomsen, J. L., Primdahl, S., Dyreborg, U. & Andersen, J. A. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br. J. Cancer 56, 814–819 (1987).
Black, W. C. & Welch, H. G. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N. Engl. J. Med. 328, 1237–1243 (1993).
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K. & Ossowski, L. Urokinase receptor and fibronectin regulate the ERKMAPK to p38MAPK activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell 12, 863–879 (2001).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERKMAPK activity as a determinant of tumor growth and dormancy; regulation by p38SAPK. Cancer Res. 63, 1684–1695 (2003). Demonstrated that a high ERK/p38 ratio favours tumour growth, whereas a high p38/ERK ratio induces tumour dormancy in vivo.
Aguirre-Ghiso, J. A., Ossowski, L. & Rosenbaum, S. GFP tagging of ERK and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004).
Luo, S. & Lee, A. S. Requirement of the p38 mitogen-activated protein kinase signalling pathway for the induction of the 78 kDa glucose-regulated protein/immunoglobulin heavy-chain binding protein by azetidine stress: activating transcription factor 6 as a target for stress-induced phosphorylation. Biochem. J. 366, 787–795 (2002).
Wang, X. Z. & Ron, D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 272, 1347–1349 (1996).
Brewer, J. W. & Diehl, J. A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl Acad. Sci. USA 97, 12625–12630 (2000).
Breckenridge, D. G., Germain, M., Mathai, J. P., Nguyen, M. & Shore, G. C. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22, 8608–8618 (2003).
Rao, R. V. et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J. Biol. Chem. 277, 21836–21842 (2002).
Ron, D. & Habener, J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes. Dev. 6, 439–453 (1992).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y. & Holbrook, N. J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 (2001).
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001).
Zong, W. X. et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 162, 59–69 (2003).
Ito, Y. et al. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 21, 6233–6242 (2001).
Mattson, M. P. et al. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 23, 222–229 (2000).
Carlberg, M. et al. Short exposures to tunicamycin induce apoptosis in SV40-transformed but not in normal human fibroblasts. Carcinogenesis 17, 2589–2596 (1996).
Wang, H. G. et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284, 339–343 (1999).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 (2000).
Morishima, N., Nakanishi, K., Takenouchi, H., Shibata, T. & Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294 (2002).
Rao, R. V. et al. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 276, 33869–33874 (2001).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Soung, Y. H. et al. Somatic mutations of CASP3 gene in human cancers. Hum. Genet. 115, 112–115 (2004).
Pommier, Y., Sordet, O., Antony, S., Hayward, R. L. & Kohn, K. W. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23, 2934–2949 (2004).
Ledoux, S., Yang, R., Friedlander, G. & Laouari, D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 63, 7284–7290 (2003). First paper to link activation of the UPR in a cultured tumour cell line to the upregulation of efflux mechanisms involved in multiple chemotherapy drug resistance.
Wang, J. C. DNA topoisomerases. Annu. Rev. Biochem. 65, 635–692 (1996).
Osheroff, N. DNA topoisomerases. Biochim. Biophys. Acta 1400, 1–2 (1998).
Chen, A. Y. & Liu, L. F. DNA topoisomerases: essential enzymes and lethal targets. Annu. Rev. Pharmacol. Toxicol. 34, 191–218 (1994).
Nitiss, J. L. & Beck, W. T. Antitopoisomerase drug action and resistance. Eur. J. Cancer 32A, 958–966 (1996).
Li, T. K. & Liu, L. F. Tumor cell death induced by topoisomerase-targeting drugs. Annu. Rev. Pharmacol. Toxicol. 41, 53–77 (2001).
Shen, J. W., Subjeck, J. R., Lock, R. B. & Ross, W. E. Depletion of topoisomerase II in isolated nuclei during a glucose-regulated stress response. Mol. Cell. Biol. 9, 3284–3291 (1989).
Yun, J., Tomida, A., Nagata, K. & Tsuruo, T. Glucose-regulated stresses confer resistance to VP-16 in human cancer cells through a decreased expression of DNA topoisomerase II. Oncol. Res. 7, 583–590 (1995).
Gosky, D. & Chatterjee, S. Down-regulation of topoisomerase IIα is caused by up-regulation of GRP78. Biochem. Biophys. Res. Commun. 300, 327–332 (2003).
Rao, R. V. et al. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 514, 122–128 (2002).
Reddy, R. K. et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 278, 20915–20924 (2003).
Swedlow, J. R. & Hirano, T. The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11, 557–569 (2003).
Yun, J., Tomida, A., Andoh, T. & Tsuruo, T. Interaction between glucose–regulated destruction domain of DNA topoisomerase IIα and MPN domain of Jab1/CSN5. J. Biol. Chem. 279, 31296–31303 (2004). Identified a sequence on topo IIα that induces its proteasome-mediated destruction in response to glucose deprivation, which activates the UPR. This might explain the increased resistance to topo-II-targeting drugs in response to ER stress.
Lin, Z. P. et al. Prevention of brefeldin A-induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance. Cancer Res. 58, 3059–3065 (1998).
Pascoe, J. M. & Roberts, J. J. Interactions between mammalian cell DNA and inorganic platinum compounds. I. DNA interstrand cross-linking and cytotoxic properties of platinum(II) compounds. Biochem. Pharmacol. 23, 1359–1365 (1974).
Yamada, M. et al. Cellular sensitization to cisplatin and carboplatin with decreased removal of platinum-DNA adduct by glucose-regulated stress. Cancer Chemother. Pharmacol. 44, 59–64 (1999).
Chatterjee, S., Hirota, H., Belfi, C. A., Berger, S. J. & Berger, N. A. Hypersensitivity to DNA cross-linking agents associated with up-regulation of glucose-regulated stress protein GRP78. Cancer Res. 57, 5112–5116 (1997). Demonstrated a correlation between ER stress and an increased sensitivity to DNA-crosslinking agents like melphalan, BCNU and cisplatin.
Mandic, A., Hansson, J., Linder, S. & Shoshan, M. C. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J. Biol. Chem. 278, 9100–9106 (2003).
Hughes, C. S., Shen, J. W. & Subjeck, J. R. Resistance to etoposide induced by three glucose-regulated stresses in Chinese hamster ovary cells. Cancer Res. 49, 4452–4454 (1989).
Tirasophon, W., Welihinda, A. A. & Kaufman, R. J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes. Dev. 12, 1812–1824 (1998). Identified the mammalian homologue of yeast Ire1 kinase, which is ubiquitously expressed, and provided the first proof that the signalling machinery of the UPR pathway is highly conserved.
Wang, X.-Z. et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO. J. 17, 5708–5717 (1998).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 (1998).
Ma, Y. & Hendershot, L. M. Delineation of the negative feedback regulatory loop that controls protein translation during ER stress. J. Biol. Chem. 278, 34864–34873 (2003).
Ma, Y., Brewer, J. W., Diehl, J. A. & Hendershot, L. M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 318, 1351–1365 (2002).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999). Identified ATF6, a new member of the mammalian UPR pathway that is not present in yeast. It delineated the unique mechanism of activation of the ER-localized transmembrane protein during ER stress, which involves cleavage of the cytosolically disposed transcription factor domain from the ER membrane.
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000). Identified a new ER-localized caspase family member that is selectively activated during the UPR, but not during other apoptosis-inducing stress conditions.
Hitomi, J. et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 165, 347–356 (2004).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Oono, K. et al. JAB1 participates in unfolded protein responses by association and dissociation with IRE1. Neurochem. Int. 45, 765–772 (2004).
Acknowledgements
The authors wish to thank I. Boime and J. Aguirre-Ghiso for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ENDOPLASMIC RETICULUM
-
A key organelle in all eukaryotic cells and the site of synthesis, folding, assembly and quality control of proteins that are to become part of the cell membrane (for example, transmembrane receptors and other integral membrane proteins) as well as proteins that are to be secreted or 'exocytosed' from the cell (for example, digestive enzymes).
- MOLECULAR CHAPERONES
-
A family of cellular proteins that mediate the correct assembly or disassembly of nascent polypeptides and, in some cases, their assembly into oligomeric structures, but which are not components of those final structures. They probably assist assembly by preventing inappropriate interactions during folding that would result in non-functional structures.
- PROTEASOME
-
A 26S multiprotein complex that catalyses the breakdown of polyubiquitylated proteins.
- Ire1
-
An endoplasmic reticulum (ER) transmembrane protein that contains three main functional domains: the ER luminal stress-sensing domain; a cytosolic kinase domain that is activated when Ire1 dimerizes and phosphorylates in trans; and a cytosolic endonuclease domain that cleaves Hac1 mRNA in yeast or XBP1 mRNA in higher organisms.
- CONFORMATIONAL DISEASES
-
A group of heterologous disorders in which a host protein is mutated or undergoes a change in conformation that interferes with its proper maturation and results in its accumulation in the ER. The most prominent examples are Alzheimer's, Parkinson's and Creutzfeldt–Jakob diseases.
- XBP1
-
A transcription factor, the mRNA of which is a substrate of the endonuclease activity of Ire1. Under non-stressed conditions, XBP1 protein levels are very low. During endoplasmic reticulum stress, 26 bases are excised from the XBP1 mRNA by Ire1, resulting in a frameshift. The new reading frame encodes a stronger transactivation domain. The transcription of XBP1 is also increased during ER stress because of the transactivation of its promoter by ATF6.
- ATF6
-
An endoplasmic reticulum (ER) transmembrane protein that contains an ER luminal stress-sensing domain and a cytosolic domain that encodes a transcription factor. During ER stress, BiP dissociates from the luminal domain of ATF6 and allows it to traffick to the Golgi, where it is cleaved by two proteases, S1P and S2P. The cytosolic transcription-factor domain is released and migrates to the nucleus, where it transactivates downstream promoters.
- CHOP
-
A transcription factor that is homologous to C/EBP. It homodimerizes to activate specific downstream targets, heterodimerizes with C/EBP family members to alter their transactivation activity, or heterodimerizes with non-C/EBP family members to activate brand new targets. CHOP has been implicated in the apoptotic event occurring during ER stress.
- PERK
-
An endoplasmic reticulum (ER) transmembrane protein that contains an ER luminal stress-sensing domain and a cytosolic kinase domain that, on activation, phosphorylates eIF-2α, resulting in general protein synthesis inhibition.
- eIF-2α
-
The smallest subunit of eIF-2, which recruits Met-tRNAi to the mRNA–40S ribosome complex when bound to GTP. Phosphorylation of this subunit during stress inhibits the assembly of the eIF-2 initiation complex, thereby blocking general protein synthesis. The phosphorylation state of eIF-2α is regulated by a family of kinases including PERK, PKR, GCN2 and HRI.
- CASPASE-12
-
Caspase-12 belongs to the group I family of caspases or ICE-like caspases, which contain a long pro-domain responsible for interaction with various adaptor proteins (CARD domain) and two catalytic subunits: p20 and p10. The activation of caspase-12 seems to be restricted to the UPR and is not activated during apoptosis induced by serum deprivation, tumour-necrosis factor, cyclohexamide or anti-FAS treatment.
- POSITRON-EMISSION TOMOGRAPHY
-
Tomographic imaging technique that detects nuclides as they decay by positron emission. The emitted positrons collide with a free electron, resulting in the conversion of matter to two γ-rays, which emerge in opposite directions.
- DORMANCY
-
Dormancy refers to cancer cells that have exited cell cycle and do not grow for prolonged periods of time in the host, but which remain viable and can be activated to re-enter the cell cycle if the appropriate growth signals are received. In experiments, tumours deprived of angiogenesis remained dormant, whereas the acquisition of a blood supply led to rapid logarithmic growth.
- MITOGEN-ACTIVATED PROTEIN KINASES
-
A group of serine/threonine kinases that mediate the response of cells to many extracellular stimuli, such as cytokines and growth factors. These protein kinases include the extracellular signal-regulated protein kinases and two stress-activated protein kinases, the c-JUN N-terminal kinases and the p38 mitogen-activated protein kinase. Activation of these kinases can lead to proliferative or apoptotic signalling.
- REACTIVE OXYGEN SPECIES
-
Highly reactive chemical radicals that are generated as products of oxygen degradation.
Rights and permissions
About this article
Cite this article
Ma, Y., Hendershot, L. The role of the unfolded protein response in tumour development: friend or foe?. Nat Rev Cancer 4, 966–977 (2004). https://doi.org/10.1038/nrc1505
Issue Date:
DOI: https://doi.org/10.1038/nrc1505
This article is cited by
-
Prolyl 4-hydroxylase subunit beta (P4HB) could serve as a prognostic and radiosensitivity biomarker for prostate cancer patients
European Journal of Medical Research (2023)
-
Oridonin promotes endoplasmic reticulum stress via TP53-repressed TCF4 transactivation in colorectal cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
The unfolded protein response (UPR) pathway: the unsung hero in breast cancer management
Apoptosis (2023)
-
Potent carotenoid astaxanthin expands the anti-cancer activity of cisplatin in human prostate cancer cells
Journal of Natural Medicines (2023)
-
Stanniocalcin 2 (STC2): a universal tumour biomarker and a potential therapeutical target
Journal of Experimental & Clinical Cancer Research (2022)