Abstract
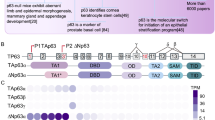
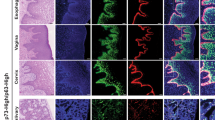
The role of p53 as a tumour suppressor is generally attributed to its ability to stop the proliferation of precancerous cells by inducing cell-cycle arrest or apoptosis. The relatives and evolutionary predecessors of p53 — p63 and p73 — share the tumour-suppressor activity of p53 to some extent, but also have essential functions in embryonic development and differentiation control. Recent evidence indicates that these ancestral functions in differentiation control contribute to the tumour-suppressor activity that the p53 family is famous for.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Flores, E. R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Almog, N. & Rotter, V. Involvement of p53 in cell differentiation and development. Biochim. Biophys. Acta 1333, F1–F27 (1997).
Lin, T. et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nature Cell Biol. 7, 165–171 (2005).
Koster, M. I., Kim, S., Mills, A. A., DeMayo, F. J. & Roop, D. R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18, 126–131 (2004).
Candi, E. et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ. 13, 1037–1047 (2006).
De Laurenzi, V. et al. Induction of neuronal differentiation by p73 in a neuroblastoma cell line. J. Biol. Chem. 275, 15226–15231 (2000).
Cordenonsi, M. et al. Links between tumor suppressors: p53 is required for TGF-β gene responses by cooperating with Smads. Cell 113, 301–314 (2003).
Hüttinger-Kirchhof, N. et al. The p53 family inhibitor δNp73 interferes with multiple developmental programs. Cell Death Differ. 13, 174–177 (2006).
Erster, S., Palacios, G., Rosenquist, T., Chang, C. & Moll, U. M. Deregulated expression of DeltaNp73α causes early embryonic lethality. Cell Death Differ. 13, 170–173 (2006).
Fontemaggi, G. et al. δEF1 repressor controls selectively p53 family members during differentiation. Oncogene 24, 7273–7280 (2005).
Cam, H. et al. p53 family members in myogenic differentiation and rhabdomyosarcoma development. Cancer Cell 10, 281–293 (2006).
Calabretta, B. & Perrotti, D. The biology of CML blast crisis. Blood 103, 4010–4022 (2004).
Ito, T. et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 52, 1369–1371 (1992).
Nanni, P. et al. Development of rhabdomyosarcoma in HER-2/neu transgenic p53 mutant mice. Cancer Res. 63, 2728–2732 (2003).
Porrello, A. et al. p53 regulates myogenesis by triggering the differentiation activity of pRb. J. Cell Biol. 151, 1295–1304 (2000).
Keller, C. et al. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev. 18, 2614–2626 (2004).
Fleischmann, A., Jochum, W., Eferl, R., Witowsky, J. & Wagner, E. F. Rhabdomyosarcoma development in mice lacking Trp53 and Fos: tumor suppression by the Fos protooncogene. Cancer Cell 4, 477–482 (2003).
Flores, E. R. et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7, 363–373 (2005).
Keyes, W. M. et al. p63 heterozygous mutant mice are not prone to spontaneous or chemically induced tumors. Proc. Natl Acad. Sci. USA 103, 8435–8440 (2006).
Moll, U. M. & Slade, N. p63 and p73: roles in development and tumor formation. Mol. Cancer Res. 2, 371–386 (2004).
Stiewe, T. & Pützer, B. M. Role of p73 in malignancy: tumor suppressor or oncogene? Cell Death Differ. 9, 237–245 (2002).
Gaiddon, C., Lokshin, M., Ahn, J., Zhang, T. & Prives, C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol. Cell Biol. 21, 1874–1887 (2001).
Lang, G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004).
Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P. & Ellisen, L. W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 (2006).
Casciano, I. et al. Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 9, 246–251 (2002).
Nakagawara, A. & Ohira, M. Comprehensive genomics linking between neural development and cancer: neuroblastoma as a model. Cancer Lett. 204, 213–224 (2004).
Scotting, P. J., Walker, D. A. & Perilongo, G. Childhood solid tumours: a developmental disorder. Nature Rev. Cancer 5, 481–488 (2005).
Saifudeen, Z. et al. Spatiotemporal switch from ΔNp73 to TAp73 isoforms during nephrogenesis: impact on differentiation gene expression. J. Biol. Chem. 280, 23094–23102 (2005).
Van Maerken, T. et al. Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma. Cancer Res. 66, 9646–9655 (2006).
Bourdon, J. C. et al. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19, 2122–2137 (2005).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
Sah, V. P. et al. A subset of p53-deficient embryos exhibit exencephaly. Nature Genet. 10, 175–180 (1995).
Rotter, V. et al. Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc. Natl Acad. Sci. USA 90, 9075–9079 (1993).
Yang, A. et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404, 99–103 (2000).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Mills, A. A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Acknowledgements
I thank the many colleagues who have contributed to these ideas, the members of my lab, and M. Schön for critical reading of the manuscript. I apologize to all colleagues whose work, although relevant, could not be cited owing to space constraints. The work was supported by grants from the Deutsche Forschungsgemeinschaft and the Deutsche Krebshilfe.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Stiewe, T. The p53 family in differentiation and tumorigenesis. Nat Rev Cancer 7, 165–167 (2007). https://doi.org/10.1038/nrc2072
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2072
This article is cited by
-
Partial p53 reactivation is sufficient to induce cancer regression
Journal of Experimental & Clinical Cancer Research (2022)
-
p73 isoforms meet evolution of metastasis
Cancer and Metastasis Reviews (2022)
-
pTINCR microprotein promotes epithelial differentiation and suppresses tumor growth through CDC42 SUMOylation and activation
Nature Communications (2022)
-
BCL11B suppresses tumor progression and stem cell traits in hepatocellular carcinoma by restoring p53 signaling activity
Cell Death & Disease (2020)
-
An insight into the anticancer mechanism of Tribulus terrestris extracts on human breast cancer cells
3 Biotech (2019)