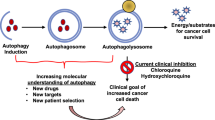
Key Points
-
Autophagy is a cellular self-cannibalization process that captures and digests cellular proteins and organelles in lysosomes.
-
Autophagy levels are normally low but are dramatically induced by starvation and stress.
-
Recycling of cellular material by autophagy sustains cellular and mammalian metabolism necessary for survival in starvation.
-
The elimination of damaged proteins and organelles by autophagy is required for cellular homeostasis.
-
Autophagy can be tumour suppressive by preventing chronic tissue damage and cancer initiation.
-
Autophagy is induced in and required for the survival of tumour cells in hypoxic tumour regions.
-
Many cancer cells upregulate autophagy that is required to support metabolism, tumorigenesis and survival to therapy.
-
In aggressive cancers, autophagy inhibition may be therapeutically advantageous.
Abstract
Autophagy (also known as macroautophagy) captures intracellular components in autophagosomes and delivers them to lysosomes, where they are degraded and recycled. Autophagy can have two functions in cancer. It can be tumour suppressive through the elimination of oncogenic protein substrates, toxic unfolded proteins and damaged organelles. Alternatively, it can be tumour promoting in established cancers through autophagy-mediated intracellular recycling that provides substrates for metabolism and that maintains the functional pool of mitochondria. Therefore, defining the context-specific role for autophagy in cancer and the mechanisms involved will be important to guide autophagy-based therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22, 132–139 (2010).
Rabinowitz, J. D. & White, E. Autophagy and metabolism. Science 330, 1344–1348 (2010).
Mathew, R. & White, E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr. Opin. Genet. Dev. 21, 113–119 (2011).
Clague, M. J. & Urbe, S. Ubiquitin: same molecule, different degradation pathways. Cell 143, 682–685 (2010).
Mizushima, N. Autophagy: process and function. Genes Dev. 21, 2861–2873 (2007).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Kirkin, V., McEwan, D. G., Novak, I. & Dikic, I. A role for ubiquitin in selective autophagy. Mol. Cell 34, 259–269 (2009).
Mathew, R. et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062–1075 (2009). Proteomics were used to identify proteins that accumulate in autophagy-defective tumour cells, one of which is p62 that is required for tumorigenesis.
Hidvegi, T. et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z. and reduces hepatic fibrosis. Science 329, 229–232 (2010).
Komatsu, M. et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature Cell Biol. 12, 213–223 (2010). This paper identifies p62 accumulation in autophagy-defective cells and KEAP1 interaction that activates NRF2.
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 (2007). This paper identifies that p62 accumulation in autophagy-defective mouse liver is required for inclusion body formation and hepatic toxicity.
Ding, W. X. et al. Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol. Cancer Ther. 8, 2036–2045 (2009).
Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl Acad. Sci. USA 100, 15077–15082 (2003). This paper describes the requirement for Becn1 in mouse development and that alleic loss renders mice tumour prone.
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003). This paper describes the requirement for Becn1 in mouse development and that alleic loss renders mice tumour prone.
Takamura, A. et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795–800 (2011). This paper demonstrates that Atg5 or Atg7 deficiency in mice causes hepatoma development that requires p62.
Aita, V. M. et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59, 59–65 (1999).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999). This paper reports a role for beclin 1 in autophagy and in inhibiting tumorigenesis when overexpressed.
He, C. & Levine, B. The Beclin 1 interactome. Curr. Opin. Cell Biol. 22, 140–149 (2010).
Youle, R. J. & Narendra, D. P. Mechanisms of mitophagy. Nature Rev. Mol. Cell Biol. 12, 9–14 (2010).
Cesari, R. et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc. Natl Acad. Sci. USA 100, 5956–5961 (2003).
Fujiwara, M. et al. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene 27, 6002–6011 (2008).
Lau, A. et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell Biol. 30, 3275–3285 (2010). This paper demonstrates that p62 binds and inhibits KEAP1 thereby pronoting NRF2 activation in autophagy-defective cells.
Villeneuve, N. F., Lau, A. & Zhang, D. D. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal 13, 1699–1712 (2010).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Duran, A. et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell 13, 343–354 (2008). This paper demonstrates that p62 deficiency prevents KRAS lung tumorigenesis.
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011). This paper reports autophagy addiction of RAS-driven cancers, specifically that RAS activation upregulates basal autophagy that is required for mitochondrial function and tumorigenesis.
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011). This paper demonstrates that NRF2 is required for KRAS and BRAF lung tumorigenesis.
Hayes, J. D. & McMahon, M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 34, 176–188 (2009).
Shen, S., Kepp, O. & Kroemer, G. The end of autophagic cell death? Autophagy 8, 1–3 (2012).
Turcotte, S. et al. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell 14, 90–102 (2008).
Elgendy, M., Sheridan, C., Brumatti, G. & Martin, S. J. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol. Cell 42, 23–35 (2011).
Moscat, J. & Diaz-Meco, M. T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137, 1001–1004 (2009).
Duran, A. et al. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 44, 134–146 (2011).
Karantza-Wadsworth, V. et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 21, 1621–1635 (2007). This paper describes that autophagy prevents genome damage and promotes tumour cell survival in a model of mammary cancer.
Mathew, R. et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 21, 1367–1381 (2007). Autophagy defects cause activation of the DNA damage response, DNA copy number variations and an elevated mutation rate, suggesting that autophagy suppresses genome instability to limit tumorigenesis.
Sakurai, T. et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 14, 156–165 (2008).
Sun, B. & Karin, M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene 27, 6228–6244 (2008).
Degenhardt, K. et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10, 51–64 (2006). Autophagy is induced in hypoxic tumour regions and is required for tumour cell survival and for limiting inflammation.
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008). Atg16l1 is required in Paneth cells to prevent an injury response and may have a role in Crohn's disease.
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009).
Chen, H. Y. & White, E. Role of autophagy in cancer prevention. Cancer Prev. Res. (Phila) 4, 973–983 (2011).
Mathew, R., Karantza-Wadsworth, V. & White, E. Role of autophagy in cancer. Nature Rev. Cancer 7, 961–967 (2007).
Wei, H., Gan, B., Wu, X. & Guan, J. L. Inactivation of FIP200 leads to inflammatory skin disorder, but not tumorigenesis, in conditional knock-out mouse models. J. Biol. Chem. 284, 6004–6013 (2009).
Wei, H. et al. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 25, 1510–1527 (2011).
Blagosklonny, M. V. Linking calorie restriction to longevity through sirtuins and autophagy: any role for TOR. Cell Death Dis. 1, e12 (2010).
He, C. et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481, 511–515 (2012).
Masiero, E. et al. Autophagy is required to maintain muscle mass. Cell. Metab. 10, 507–515 (2009).
Suzuki, S. W., Onodera, J. & Ohsumi, Y. Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS ONE 6, e17412 (2011). Autophagy is required in yeast to prevent mitochondrial inpairment and cell death.
Onodera, J. & Ohsumi, Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J. Biol. Chem. 280, 31582–31586 (2005).
Kamada, Y., Sekito, T. & Ohsumi, Y. Autophagy in yeast: a TOR-mediated response to nutrient starvation. Curr. Top. Microbiol Immunol. 279, 73–84 (2004).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004). Autophagy promotes survival of mice during neonatal starvation.
Tsukamoto, S. et al. Autophagy is essential for preimplantation development of mouse embryos. Science 321, 117–120 (2008).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005). Autophagy enables long-term survival of lymphoid cells to growth factor deprivation.
Mizushima, N. Methods for monitoring autophagy using GFP-LC3 transgenic mice. Methods Enzymol. 452, 13–23 (2009).
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 (2004).
Lock, R. et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol. Biol. Cell 22, 165–178 (2010). RAS upregulates autophagy that promotes transformation.
Yang, S. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 (2011). This paper reports that pancreatic cancers display autophagy addiction with high basal autophagy that is required for growth, survival and tumorigenesis.
Wu, J. J. et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 1, 425–437 (2009).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Valentin-Vega, Y. A. et al. Mitochondrial dysfunction in ataxia telangiectasia. Blood 119, 1490–1500 (2011).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Chun, S. Y. et al. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1alpha and HIF-2alpha target genes. Mol. Cancer 9, 293 (2010).
Semenza, G. L. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 20, 51–56 (2010).
Chen, C., Pore, N., Behrooz, A., Ismail-Beigi, F. & Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 276, 9519–9525 (2001).
Zheng, B. et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 33, 237–247 (2009).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11, 407–420 (2007).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011).
Kon, M. et al. Chaperone-mediated autophagy is required for tumor growth. Sci. Transl. Med. 3, 109ra117 (2011).
Amaravadi, R. K. et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 17, 654–666 (2011).
Garber, K. Inducing indigestion: companies embrace autophagy inhibitors. J. Natl Cancer Inst. 103, 708–710 (2011).
White, E. & DiPaola, R. S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 15, 5308–5316 (2009).
Maycotte, P. et al. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8, 200–212 (2012).
Sheen, J. H., Zoncu, R., Kim, D. & Sabatini, D. M. Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell 19, 613–628 (2011). Autophagy induction by leucine starvation is required for the survival of melanomas.
Feldman, M. E. & Shokat, K. M. New inhibitors of the PI3K-Akt-mTOR pathway: insights into mTOR signaling from a new generation of Tor Kinase Domain Inhibitors (TORKinibs). Curr. Top. Microbiol Immunol. 347, 241–262 (2010).
Altman, B. J. et al. Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis. Oncogene 30, 1855–1867 (2011).
Amaravadi, R. K. et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 117, 326–336 (2007).
Bellodi, C. et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest. 119, 1109–1123 (2009).
Carew, J. S. et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110, 313–322 (2007).
Degtyarev, M. et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol. 183, 101–116 (2008).
Fan, Q. W. et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci. Signal 3, ra81 (2010).
Han, W. et al. EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS ONE 6, e18691 (2011).
Ma, X. H. et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 17, 3478–3489 (2011).
Maclean, K. H., Dorsey, F. C., Cleveland, J. L. & Kastan, M. B. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J. Clin. Invest. 118, 79–88 (2008).
Pan, Y. et al. Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy. Clin. Cancer Res. 17, 3248–3258 (2011).
Parkhitko, A. et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc. Natl Acad. Sci. USA 108, 12455–12460 (2011).
Saleem, A. et al. Effect of dual inhibition of apoptosis and autophagy in prostate cancer. Prostate 12 Jan 2012 (doi:10.1002/pros.22487).
Shi, Y. H. et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 7, 1159–1172 (2011).
Wu, Z. et al. Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer 1, 40–49 (2010).
Fung, C., Lock, R., Gao, S., Salas, E. & Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 19, 797–806 (2008).
Mortensen, M. et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 208, 455–467 (2011).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011). Autophagy is required to promote antitumour immunity in response to cytotoxic chemotherapy.
Eng, C. H., Yu, K., Lucas, J., White, E. & Abraham, R. T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal 3, ra31 (2010). Ammonia produced from glutaminolysis induces autophagy.
Cheong, H., Lindsten, T., Wu, J., Lu, C. & Thompson, C. B. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc. Natl Acad. Sci. USA 108, 11121–11126 (2011).
Moscat, J. & Diaz-Meco, M. T. p62: a versatile multitasker takes on cancer. Trends Biochem. Sci. 14 Mar 2012 (doi:10.1016/j.tibs.2012.02.008).
Acknowledgements
I thank members of the White laboratory for helpful discussions and advice. This work was supported by grants from the US National Institutes of Health (R37 CA53370, RO1 CA130893 and RC1 147961), DOD (W81XWH06-1-0514 and W81XWH05) and the V Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Tricarboxylic acid (TCA) cycle
-
Also known as the citric acid cycle or the Krebs cycle, the TCA cycle is a series of chemical reactions that generate energy and building blocks through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and water.
- Reactive oxygen species
-
(ROS). Chemically reactive molecules containing oxygen that cause cellular damage or that activate signalling.
- Steatohepatitis
-
A pathological condition, also known as fatty liver disease, characterized by inflammation and fat accumulation in the liver.
- Proteasome
-
A large protein complex responsible for the degradation of soluble proteins.
- NF-κB
-
A transcription factor that controls the immune response to damage and infection.
- mTOR
-
A serine/threonine protein kinase that regulates cell growth, cell proliferation, cell survival, protein synthesis and transcription in response to nutrient and growth factor availability.
- Hypoxia
-
A pathological condition in which the body as a whole or a region of the body, such as a tumour, is deprived of an adequate oxygen supply.
- Cataplerosis
-
The process by which metabolic intermediates are removed from metabolic pathways.
- Glycolysis
-
The metabolic pathway that converts glucose into pyruvate and in the process produces ATP and reduced NADH.
- Glutaminolysis
-
A series of biochemical reactions in which the amino acid glutamine is degraded to glutamate then to α-ketoglutarate for further metabolism in the tricarboxylic acid cycle.
- Anaplerosis
-
The process of replenishment of depleted metabolic cycle or pathway intermediates.
- ER stress
-
A stress adaptation pathway activated by the accumulation of unfolded proteins in the endoplasmic reticulum (ER).
Rights and permissions
About this article
Cite this article
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12, 401–410 (2012). https://doi.org/10.1038/nrc3262
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3262
This article is cited by
-
PSMD2 contributes to the progression of esophageal squamous cell carcinoma by repressing autophagy
Cell & Bioscience (2023)
-
Regulation of phosphoglycerate kinase 1 and its critical role in cancer
Cell Communication and Signaling (2023)
-
Nanomedicine for autophagy modulation in cancer therapy: a clinical perspective
Cell & Bioscience (2023)
-
MGCG regulates glioblastoma tumorigenicity via hnRNPK/ATG2A and promotes autophagy
Cell Death & Disease (2023)
-
Cezanne promoted autophagy through PIK3C3 stabilization and PIK3C2A transcription in lung adenocarcinoma
Cell Death Discovery (2023)