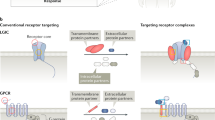
Key Points
-
The concepts that synthetic agonists need mimic natural agonists and that all antagonists must simply preclude agonist activation of receptors is challenged.
-
Previous ideas of linear efficacy whereby receptor activation by an agonist necessarily leads to initiation of all GPCR behaviors such as desensitization, phosphorylation, internalization and dimerization have given way in light of evidence to show that efficacy can be 'collateral', that is, some but not all GPCR behaviors can be produced by some agonists. Examples are given of signaling collateral efficacy where agonists preferentially activate some cellular pathways but not others (stimulus trafficking).
-
This idea naturally extends to concepts of how therapeutic targets should be screened for new molecular leads. Specifically, different types of assays might uncover different lead activity in the same library of compounds.
-
Evidence is presented that allosteric antagonists can be 'permissive', in that they block the signaling of some agonists but not others. The extension of this idea to the blockade of some GPCR-mediated pathological functions but not normal physiological functions leads to reconsideration of tractable therapeutic targets for GPCR antagonists.
-
The unique properties of permissive antagonists are discussed using the example of the new CCR5 allosteric HIV-1 entry inhibitor aplaviroc.
-
The distinction between the geometrical location of allosteric binding sites and allosteric effect is drawn; specifically, different allosteric effects can be produced by binding at a common allosteric site much like agonists have different efficacies binding to a single agonist binding site. In this case, 'efficacy' for allosteric antagonists is equated to the cooperativity constants for affinity and efficacy for GPCR agonist activation.
Abstract
New perspectives on the complexity of G-protein-coupled receptor (GPCR) signalling and the increased resolution of existing tools for studying GPCR behaviour has led to the conception of new hypotheses that affect the discovery of drugs acting at GPCRs. Taking into consideration the novel concepts of collateral efficacy and permissive antagonism in the search for synthetic agonists and antagonists, respectively, will be essential in the search for drugs with unique therapeutic profiles. Here, the design of drugs against HIV is used as an example of how these concepts might be taken into consideration for GPCR-targeted drugs in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kenakin, T. P. Efficacy at G-protein-coupled receptors. Nature Rev. Drug Discov. 1, 103–109 (2002).
Stephenson, R. P. A modification of receptor theory Br. J. Pharmacol. 11, 379–393 (1956). The classic paper defining efficacy as a property separate from affinity for agonists.
Brady, A. E. & Limbird, L. E. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cellular Signalling. 14, 297–309 (2002). Concisely summarizes the growing number of proteins that interact with GPCRs in the cell membrane beyond G-proteins.
Lanier, S. M. AGS proteins, GPR motifs and the signals processed by heterotrimeric G proteins. Biol. Cell. 96, 369–372 (2004).
George, S. R., O'Dowd, B. F. & Lee, S. P. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nature Rev. Drug Discov. 1, 808–820 (2002).
Mack, M. et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of hiv infectivity. J. Exp. Med. 187, 1215–1224 (1998).
Kohout, T. A. & Lefkowitz, R. J. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization Mol. Pharmacol. 63, 9–18 (2003).
Sneddon, W. B. et al. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EPB50). J. Biol. Chem. 278, 43787–43796 (2003).
Ferguson, S. S. G. Evolving concepts in G-protein coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24 (2001).
Roettger, B. F. et al. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol. Pharmacol. 51, 357–362 (1997). One of the first studies to differentiate endocytosis from receptor activation.
Thomas, W. G., Quian, H., Chang, C. -S. & Karnik, S. Agonist-induced phosphorylation of the angiotensin II (AT1A) receptor requires generation of a conformation that is distinct from the inositol phosphate-signaling state. J. Biol. Chem. 275, 2893–2900 (2000).
Horne, W. C., Shyu, J. -F., Chakraborthy, M. & Baron, R. Signal transduction by calcitonin: multiple ligands, receptors and signaling pathways. Trends Endocrinol. Metab. 5, 395–401 (1994).
Kenakin, T. P. Agonist-receptor efficacy II: agonist-trafficking of receptor signals. Trends Pharmacol. Sci. 16, 232–238 (1995).
Watson, C., Chen, G., Irving, P. E., Way, J., Chen, W. -J. & Kenakin, T. P. The use of stimulus-biased assay systems to detect agonist-specific receptor active states: Implications for the trafficking of receptor stimulus by agonists. Mol. Pharmacol. 58, 1230–1238 (2000).
Kenakin, T. P. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol. Sci. 24, 346–354 (2003).
Kenakin, T. P. Efficacy at G protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 42, 349–379 (2002).
Burgen, A. S. V. Conformational changes and drug action. Fed. Proc. 40, 2723–2728 (1966).
Kenakin, T. P. & Onaran, O. The ligand paradox between affinity and efficacy: can you be there and not make a difference? Trends Pharmacol. Sci. 23, 275–280 (2002).
Hoffmann, C. et al. A FlAsH FRET approach to determine G protein-coupled receptor activation in living cells. Nature Methods 2, 171–176 (2005).
Gales, C. et al. Real-time monitoring of receptor/G-protein interactions in living cells. Nature Methods 2, 177–184 (2005).
Jakubic, J., Bacakova, L., Lisa, V., El-Fakahany, E. E. & Tucek, S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol. Pharmacol. 52, 172–179 (1997).
Feng, Y., Broder, C. C., Kennedy, P. E. & Berger, E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272, 872–877 (1996).
Sachpatzidis, A. et al. Identification of allosteric peptide agonists of CXCR4. J. Biol. Chem. 278, 896–907 (2003). One of the first accounts of how permissive antagonists might spare some agonist signalling.
Nagasaw, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Tachibana, K. et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393, 591–594 (1998).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Demarest, J. et al. Single and multiple dose escalation study to investigate the safety, pharmacokinetics, and receptor binding of 873140, a novel CCR5 receptor antagonist, in healthy subjects. 11th Conf. Retroviruses Opportunistic Infect. (San Francisco, Abs. 139, 2004).
Demarest, J., Shibayama S., Ferris, R., Vavro, C., StClair, M. & L. Boone. L. A novel CCR5 antagonist, 873140, exhibits potent in vitro anti-HIV activity. XV International AIDS Conf. Bangkok Intersci. Conf. on Antimicrobial Agents Chemother. (Abs. WeOrA1231, 2004).
Lalezari, J. et al. Antiviral activity and safety of 873140, a novel CCR5 antagonist, during short-term monotheapy in HIV-infected adults. AIDS 19, 1443–1448 (2005)
Watson, C., Jenkinson, S., Kazmierski., W. & Kenakin, T. P. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric non-competitive HIV entry-inhibitor. Mol. Pharmacol. 67, 1268–1282 (2005). Shows, in quantitative terms, how the unique properties of the new CCR5 HIV entry inhibitors can be described.
Maeda. K. et al. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 78, 8654–8662 (2004). Highlights some of the interesting characteristics of the new CCR5 HIV entry inhibitors.
Tsamis, F. et al. Analysis of the mechanism by which the small molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type-1 entry. J. Virol. 77, 5201–5208 (2003).
Wu, L. et al. Interaction of the chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186, 1373–1381 (1997).
Blanpain, C. et al. The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 278, 5179–5187 (2003).
Wyatt, R. & Sodroski, J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280, 1884–1888 (1988).
Poignard, P., Saphire, E. O., Parren, P. W. & Burton, D. R. gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19, 253–274 (2001).
Trkola, A. et al. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl Acad. Sci. USA 99, 395–400 (2002).
Kuhmann, S. E. et al. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape form small-molecule CCR inhibitor J. Virol. 78, 2790–2807 (2004).
Alkhatib, G., Locati, M., Kennedy, P. E., Murphy, P. M. & Berger, E. A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G-protein signaling and importance of coreceptor down-modulation. Virology 234, 340–349 (1997).
Mack, M. et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187, 1215–1224 (1998).
Signoret, N., Pelchen-Mathews, A., Mack, M., Proudfoot, A. E. & Marsh, M. Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 151, 1281–1294 (2000).
Signoret, N. et al. Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent. Mol. Biol. Cell 16, 902–917 (2005).
Garzino-Demo, A. et al. Spontaneous and antigen-induced production of HIV-inhibitory β-chemokines are associated with AIDS-free status Proc. Natl Acad. Sci USA 96, 11986–11991 (1999).
Ullum, H. et al. Production of β-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein-1β are associated with a decreased risk of HIV risk progression. J. Infect. Dis. 177, 331–336 (1998).
Sabroe, I. et al. A small molecule antagonist of chemokine receptors CCR1 and CCR3. J. Biol. Chem. 275, 25985–25992 (2000).
Bartus, R. T., Dean, R. L., Beer, B. & Lippa, A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–417 (1982).
Pommier, Y. & Cherfils, J. Interfacial inhibition of macromolecular interactions: Nature's paradigm for drug discovery. Trends Pharmacol. Sci. 26, 138–145 (2005).
Kunkel, E. J. et al. An integrative biology approach for analysis of drug action in models of human vascular inflammation. FASEB J. 18, 1279–1281 (2004).
Kunkel, E. J. et al. Rapid structure–activity and selectivity analysis of kinase inhibitors by BioMap analysis in complex human primary cell-based models. Assay Drug Dev. Technol. 2, 431–441 (2004).
Ehlert,. F J. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharmacol. 33, 187–194 (1988). The classic paper describing the simple model of allosterism for GPCRs.
Black, J. W. & Leff, P. Operational models of pharmacological agonists. Proc. Roy. Soc. Lond., B, (Biol.) 220, 141–162 (1983).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- RECEPTOR SUBTYPE
-
Major endogenous hormones and neurotransmitters activate families of receptors that are related by a general affinity for the endogenous agonist but which nevertheless can be distinguished by antagonists and synthetic agonists.
- RECOMBINANT
-
DNA containing new genetic material is placed in surrogate cells that then express the relevant protein. Genetic engineering can be used to do this deliberately to produce receptor systems in surrogate cell lines for experimental testing.
- PERMISSIVE ANTAGONISM
-
This term distinguishes allosteric modulators that demonstrate probe dependence — that is, allosteric modulators that block the response to some agonists but not others. Although all permissive antagonists must be allosteric, not all allosteric antagonists need be permissive.
- EFFICACY
-
Historically, this term was given to agonists to define the property of the molecule that causes the production of physiological response. However, with the discovery of negative efficacy (inverse agonists) and efficacy related to other properties of receptors that do not involve physiological response, a more general definition of efficacy is that property of a molecule that causes the receptor to change its behaviour toward the host cell.
- RECEPTOR OLIGOMERS (DIMERS)
-
GPCRs form large associated units comprising two receptors of the same type (homodimers), two different receptors (heterodimers) or multiple receptors (oligomers). These units can have new reactivity profiles with respect to ligands and cells.
- INTERNALIZATION (ENDOCYTOSIS)
-
The natural process by which receptors are assimilated back into the cytosol of the cell to be degraded or processed and shuttled back to the cell surface.
- COLLATERAL EFFICACY
-
Defining 'efficacy' as active biological results of ligand binding, GPCR drugs often have many efficacies resulting in changes in GPCR behaviour. Whereas 'linear' efficacy describes all such behaviours emanating from a single receptor binding event, 'collateral' efficacy describes selective not all-inclusive behaviours resulting from the binding of some ligands.
- FLUORESCENCE RESONANCE ENERGY TRANSFER
-
(FRET). A technique that can be used to study GPCR conformational changes in living cells. This technique monitors the transfer of energy between two fluorophores bound in close proximity to each other and is sensitive to very small changes in distance (on the order of those that occur with changes in protein conformation).
- BIOLUMINESCENCE RESONANCE ENERGY TRANSFER
-
(BRET). A technique used to directly monitor receptor–G-protein interaction in real time. This method monitors energy transfer between a bioluminescent donor and a fluorescent acceptor as the two are brought together through ligand activation.
- ALLOSTERIC EFFECT
-
The imposition of an effect on a protein through interaction of a molecule with a site on the protein distinct from the natural binding locus for the endogenous ligand for that protein. The interactions between the allosteric molecule and the endogenous ligand occur through the protein and not through direct steric interaction.
- ORTHOSTERIC EFFECT
-
Effect of a ligand through steric hindrance; the binding of one ligand precludes the binding of another because they require the same binding site.
Rights and permissions
About this article
Cite this article
Kenakin, T. New Concepts in Drug Discovery: Collateral Efficacy and Permissive Antagonism. Nat Rev Drug Discov 4, 919–927 (2005). https://doi.org/10.1038/nrd1875
Issue Date:
DOI: https://doi.org/10.1038/nrd1875
This article is cited by
-
The activity of the C4-dicarboxylic acid chemoreceptor of Pseudomonas aeruginosa is controlled by chemoattractants and antagonists
Scientific Reports (2018)
-
A strategy to discover decoy chemokine ligands with an anti-inflammatory activity
Scientific Reports (2015)
-
Muscarinic acetylcholine receptors: novel opportunities for drug development
Nature Reviews Drug Discovery (2014)
-
Allosteric regulation of pathologic angiogenesis: potential application for angiogenesis-related blindness
Archives of Pharmacal Research (2014)