Key Points
-
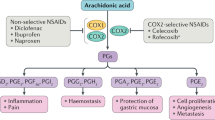
The formation of prostanoids depends on the function of cyclooxygenase (COX) enzymes that convert, through a two-step process, arachidonic acid into prostaglandin H2, the precursor of the other prostanoids.
-
COX enzymes exist in two forms: COX1, which seems to be more associated with physiological functions, and COX2, which is readily inducible and seems to be more associated with pathological functions. These enzymes are the targets for the non-steroidal anti-inflammatory drugs (NSAIDs). Work in the past 10–15 years has resulted in the production of selective inhibitors of COX2 designed to produce the same anti-inflammatory effects as the traditional NSAIDs, associated with inhibition of COX2, with less damaging effects on the stomach, associated with inhibition of COX1.
-
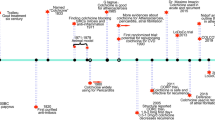
Following the introduction of selective COX2 inhibitors around 5 years ago there has been much debate as to whether these drugs are actually safer than the traditional NSAIDs they were meant to replace. In particular, it has been proposed that selective inhibition of COX2 is associated with an increased incidence of thrombotic events driven by a reduction in the formation of the antithrombotic prostanoid, prostacylin, in blood vessels. This, it has been suggested, negates any safety benefits in the gastrointestinal tract. In the autumn of 2004, the COX2-selective drug rofecoxib was withdrawn from the market because of an excess of thrombotic events in a placebo-controlled trial investigating its usefulness in the prevention of colon cancer.
-
Discussions have continued, often hampered by the lack of data for traditional NSAIDs from controlled clinical trials. Here we discuss the evidence and the latest recommendations for the use of selective inhibitors of COX2 as well as the traditional NSAIDs.
Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the formation of prostanoids by the enzyme cyclooxygenase (COX). Work in the past 15 years has shown that COX exists in two forms: COX1, which is largely associated with physiological functions, and COX2, which is largely associated with pathological functions. Heated debate followed the introduction of selective COX2 inhibitors around 5 years ago: do these drugs offer any advantages over the traditional NSAIDs they were meant to replace, particularly in regard to gastrointestinal and cardiovascular side effects? Here we discuss the evidence and the latest recommendations for the use of selective inhibitors of COX2 as well as the traditional NSAIDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vane, J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 231, 232–235 (1971).
Ferreira, S. H., Moncada, S. & Vane, J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nature New Biol. 231, 237–239 (1971).
Smith, J. B. & Willis, A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nature New Biol. 231, 235–237 (1971). References 1–3 are back-to-back papers establishing that NSAIDs have the common mechanism of action of inhibiting prostanoid formation. Reference 3 in particular showed that aspirin inhibited prostanoid formation in platelets; now known, of course, to be thromboxane A 2.
Vane, J. R., Bakhle, Y. S. & Botting, R. M. Cyclooxygenase 1 and 2. Annu. Rev. Pharmacol. Toxicol. 38, 97–120 (1998).
Smith, W. L., DeWitt, D. L. & Garavito, R. M. Cyclooxygenases: structural, cellular and molecular biology. Annu. Rev. Biochem. 69, 145–182 (2000).
DuBois, R. N. et al. Cyclooxygenase in biology and disease. FASEB J. 12, 1063–1073 (1998).
FitzGerald, G. A. & Patrono, C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 345, 433–442 (2001).
Flower, R. J. The development of COX2 inhibitors. Nature Rev. Drug Discov. 2, 179–191 (2003).
Warner, T. D. & Mitchell, J. A. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 18, 790–804 (2004).
Rosen, G. D., Birkenmeier, T. M., Raz, A. & Holtzman, M. J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem. Biophys. Res. Commun. 164, 1358–1365 (1989). Study published 2 years before the publication of definitive characterizations of COX2 showing that cell culture greatly increased the production of prostanoids and that this was associated with the appearance of a COX-related mRNA, suggesting the existence of an inducible isoform of COX.
Xie, W. L., Chipman, J. G., Robertson, D. L., Erikson, R. L. & Simmons, D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl Acad. Sci. USA 88, 2692–2696 (1991).
Kujubu, D. A., Fletcher, B. S., Varnum, B. C., Lim, R. W. & Herschman, H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 266, 12866–12872 (1991).
Hla, T. & Neilson, K. Human cyclooxygenase-2 cDNA. Proc. Natl Acad. Sci. USA 89, 7384–7388 (1992). References 11–13 report the initial definitive reports of the existence of COX2.
Masferrer, J. L., Seibert, K., Zweifel, B. & Needleman, P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc. Natl Acad. Sci. USA 89, 3917–3921 (1992).
O'Banion, M. K., Winn, V. D. & Young, D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl Acad. Sci. USA 89, 4888–4892 (1992).
Mitchell, J. A., Akarasereenont, P., Thiemermann, C., Flower, R. J. & Vane, J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl Acad. Sci. USA 90, 11693–11697 (1993). Early study demonstrating in isolated cell systems, notably including endothelial cells, that NSAIDs show differential selectivity for COX1 and COX2.
Mitchell, J. A. & Evans, T. W. Cyclooxygenase-2 as a therapeutic target. Inflamm. Res. 47 (Suppl. 2), S88–S92 (1998).
McAdam, B. F. et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl Acad. Sci. USA 96, 272–277 (1999). Clinical study that showed that dosing of healthy volunteers with celecoxib reduced urinary prostacyclin metabolites. Led to the idea that prostacyclin production by endothelial cells within the circulation is dependent on the activity of COX2.
Catella-Lawson, F. et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J. Pharmacol. Exp. Ther. 289, 735–741 (1999).
Fitzgerald, G. A. Coxibs and cardiovascular disease. N. Engl. J. Med. 351, 1709–1711 (2004).
Horton, R. Vioxx, the implosion of Merck, and aftershocks at the FDA. Lancet 364, 1995–1996 (2004).
Topol, E. J. Failing the public health — rofecoxib, Merck, and the FDA. N. Engl. J. Med. 351, 1707–1709 (2004).
Okie, S. Raising the safety bar — the FDA's coxib meeting. N. Engl. J. Med. 352, 1283–1285 (2005).
Antman, E. M., DeMets, D. & Loscalzo, J. Cyclooxygenase inhibition and cardiovascular risk. Circulation 112, 759–770 (2005).
Chandrasekharan, N. V. et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc. Natl Acad. Sci. USA 99, 13926–13931 (2002).
Simmons, D. L., Botting, R. M. & Hla, T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56, 387–437 (2004).
Schade, S. et al. Diverse functional coupling of cyclooxygenase 1 and 2 with final prostanoid synthases in liver macrophages. Biochem. Pharmacol. 64, 1227–1232 (2002).
Snipes, J. A., Kis, B., Shelness, G. S., Hewett, J. A. & Busija, D. W. Cloning and characterization of cyclooxygenase-1b (putative COX-3) in rat. J. Pharmacol. Exp. Ther. 313, 668–676 (2005).
Warner, T. D. & Mitchell, J. A. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proc. Natl Acad. Sci. USA 99, 13371–13373 (2002).
Warner, T. D. et al. Cyclooxygenases 1, 2, and 3 and the production of prostaglandin I2: investigating the activities of acetaminophen and cyclooxygenase-2-selective inhibitors in rat tissues. J. Pharmacol. Exp. Ther. 310, 642–647 (2004).
Kis, B., Snipes, J. A. & Busija, D. W. Acetaminophen and the COX-3 puzzle: sorting out facts, fictions and uncertainties. J. Pharmacol. Exp. Ther. 315, 1–7 (2005).
Warner, T. D. et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl Acad. Sci. USA 96, 7563–7568 (1999).
Mitchell, J. A. & Warner, T. D. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 128, 1121–1132 (1999).
Perini, R., Fiorucci, S. & Wallace, J. L. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can. J. Gastroenterol. 18, 229–236 (2004).
Zimmermann, K. C., Sarbia, M., Schror, K. & Weber, A. A. Constitutive cyclooxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol. Pharmacol. 54, 536–540 (1998).
Wallace, J. L., McKnight, W., Reuter, B. K. & Vergnolle, N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119, 706–714 (2000).
Tanaka, A., Hase, S., Miyazawa, T., Ohno, R. & Takeuchi, K. Role of cyclooxygenase (COX)-1 and COX-2 inhibition in nonsteroidal anti-inflammatory drug-induced intestinal damage in rats: relation to various pathogenic events. J. Pharmacol. Exp. Ther. 303, 1248–1254 (2002).
Langenbach, R. et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83, 483–492 (1995).
Sigthorsson, G. et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122, 1913–1923 (2002).
Vane, J. R. & Warner, T. D. Nomenclature for COX-2 inhibitors. Lancet 356, 1373–1374 (2000).
Moncada, S., Gryglewski, R., Bunting, S. & Vane, J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 263, 663–665 (1976). First description of prostacyclin and demonstration of its capacity to inhibit platelet aggregation.
Hamberg, M., Svensson, J. & Samuelsson, B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl Acad. Sci. USA 72, 2994–2998 (1975). First definitive characterization of the thromboxanes; formed by platelets, thromboxane A 2 drives the formation of thrombi and is the therapeutic target of low-dose aspirin.
Roth, G. J., Machuga, E. T. & Ozols, J. Isolation and covalent structure of the aspirin-modified, active-site region of prostaglandin synthetase. Biochemistry 22, 4672–4675 (1983).
Patrono, C. Aspirin: new cardiovascular uses for an old drug. Am. J. Med. 110, 62S–65S (2001).
Pedersen, A. K. & FitzGerald, G. A. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N. Engl. J. Med. 311, 1206–1211 (1984).
Murata, T. et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 388, 678–682 (1997).
Cheng, Y. et al. Role of prostacyclin in the cardiovascular response to thromboxane A2 . Science 296, 539–541 (2002).
Rocca, B. et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc. Natl Acad. Sci. USA 99, 7634–7639 (2002).
Tanaka, N., Sato, T., Fujita, H. & Morita, I. Constitutive expression and involvement of cyclooxygenase-2 in human megakaryocytopoiesis. Arterioscler. Thromb. Vasc. Biol. 24, 607–612 (2004).
Hasan, K., Warner, T. D., Vojnovic, I., Pepper, J. R. & Mitchell. Characterisation of cyclo-oxygenase activity in human megakaryocytes: relevance to platelet COX-2. British Pharmacological Society Meeting, London, UK, December, pA2 online. 2004;4:036P (2003).
Weber, A. A. et al. Flow cytometry analysis of platelet cyclooxygenase-2 expression: induction of platelet cyclooxygenase-2 in patients undergoing coronary artery bypass grafting. Br. J. Haematol. 117, 424–426 (2002).
Censarek, P. et al. Cyclooxygenase COX-2a, a novel COX-2 mRNA variant, in platelets from patients after coronary artery bypass grafting. Thromb. Haemost. 92, 925–928 (2004).
Patrignani, P. Aspirin insensitive eicosanoid biosynthesis in cardiovascular disease. Thromb. Res. 110, 281–286 (2003).
Sanderson, S., Emery, J., Baglin, T. & Kinmonth, A. L. Narrative review: aspirin resistance and its clinical implications. Ann. Intern. Med. 142, 370–380 (2005).
Belton, O., Byrne, D., Kearney, D., Leahy, A. & Fitzgerald, D. J. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 102, 840–845 (2000).
Bishop-Bailey, D. et al. Induction of cyclooxygenase-2 in human saphenous vein and internal mammary artery. Arterioscler. Thromb. Vasc. Biol. 17, 1644–1648 (1997).
Bishop-Bailey, D., Pepper, J. R., Larkin, S. W. & Mitchell, J. A. Differential induction of cyclo-oxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arterioscler. Thromb. Vasc. Biol. 18, 1655–1661 (1998).
Jimenez, R. et al. Role of Toll-like receptors 2 and 4 in the induction of cyclooxygenase-2 in vascular smooth muscle. Proc. Natl Acad. Sci. USA 102, 4637–4462 (2005).
Topper, J. N., Cai, J., Falb, D. & Gimbrone, M. A. Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl Acad. Sci. USA 93, 10417–10422 (1996). Paper demonstrating that COX2 expression can be increased in endothelial cells in culture by short-term exposure to shear forces. Taken as support of the hypothesis that endothelial cells normally express COX2 within blood vessels.
Inoue, H. et al. Transcriptional and posttranscriptional regulation of cyclooxygenase-2 expression by fluid shear stress in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 22, 1415–1420 (2002).
Doroudi, R., Gan, L. M., Selin Sjogren, L. & Jern, S. Effects of shear stress on eicosanoid gene expression and metabolite production in vascular endothelium as studied in a novel biomechanical perfusion model. Biochem. Biophys. Res. Commun. 269, 257–264 (2000).
McCormick, S. M., Whitson, P. A., Wu, K. K. & McIntire, L. V. Shear stress differentially regulates PGHS-1 and PGHS-2 protein levels in human endothelial cells. Ann. Biomed. Eng. 28, 824–833 (2000).
Dancu, M. B., Berardi, D. E., Vanden Heuvel, J. P. & Tarbell, J. M. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 2088–2094 (2004).
Okahara, K., Sun, B. & Kambayashi, J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18, 1922–1926 (1998).
Wasserman, S. M. et al. Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol. Genomics 12, 13–23 (2002).
McCormick, S. M. et al. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc. Natl Acad. Sci. USA 98, 8955–8960 (2001).
Warabi, E. et al. Effect on endothelial cell gene expression of shear stress, oxygen concentration, and low-density lipoprotein as studied by a novel flow cell culture system. Free Radic. Biol. Med. 37, 682–694 (2004).
Chen, B. P. et al. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol. Genomics 7, 55–63 (2001).
Garcia-Cardena, G., Comander, J., Anderson, K. R., Blackman, B. R. & Gimbrone, M. A. Jr. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc. Natl Acad. Sci. USA 98, 4478–4485 (2001).
Ohura, N. et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. J. Atheroscler. Thromb. 10, 304–313 (2003).
Dai, G. et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl Acad. Sci. USA 101, 14871–14876 (2004).
Brooks, A. R., Lelkes, P. I. & Rubanyi, G. M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol. Genomics 9, 27–41 (2002).
Yoshisue, H. et al. Large scale isolation of non-uniform shear stress-responsive genes from cultured human endothelial cells through the preparation of a subtracted cDNA library. Atherosclerosis 162, 323–334 (2002).
Resnick, N. & Gimbrone, M. A. Jr. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 9, 874–882 (1995).
Dekker, R. J. et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100, 1689–1698 (2002).
Baker, C. S. et al. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler. Thromb. Vasc. Biol. 19, 646–655 (1999).
Schonbeck, U., Sukhova, G. K., Graber, P., Coulter, S. & Libby, P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am. J. Pathol. 155, 1281–1291 (1999).
Stemme, V., Swedenborg, J., Claesson, H. & Hansson, G. K. Expression of cyclo-oxygenase-2 in human atherosclerotic carotid arteries. Eur. J. Vasc. Endovasc. Surg. 20, 146–152 (2000).
Lucas, R. et al. Expression of COX-1, but not COX-2 or COX-3; like immunoreactivity in human blood vessels and heart. British Pharmacological Society Meeting, London, UK, December, pA2online 2004;1(4):035P (2003).
Hamilton, L. C., Mitchell, J. A., Tomlinson, A. M. & Warner, T. D. Synergy between cyclo-oxygenase-2 induction and arachidonic acid supply in vivo: consequences for nonsteroidal anti-inflammatory drug efficacy. FASEB J. 13, 245–251 (1999).
Pratico, D., Tillmann, C., Zhang, Z. B., Li, H. & FitzGerald, G. A. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc. Natl Acad. Sci. USA 98, 3358–3363 (2001).
Csiszar, A. et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 90, 1159–1166 (2002).
Wong, E., Huang, J. Q., Tagari, P. & Riendeau, D. Effects of COX-2 inhibitors on aortic prostacyclin production in cholesterol-fed rabbits. Atherosclerosis 157, 393–402 (2001).
Rudic, R. D. et al. COX-2-derived prostacyclin modulates vascular remodeling. Circ. Res. 96, 1240–1247 (2005).
Riendeau, D. et al. Comparison of the cyclo-oxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can. J. Physiol. Pharmacol. 75, 1088–1095 (1997).
Boutaud, O., Aronoff, D. M., Richardson, J. H., Marnett, L. J. & Oates, J. A. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H2 synthases. Proc. Natl Acad. Sci. USA 99, 7130–7135 (2002).
Aronoff, D. M., Boutaud, O., Marnett, L. J. & Oates, J. A. Inhibition of prostaglandin H2 synthases by salicylate is dependent on the oxidative state of the enzymes. J. Pharmacol. Exp. Ther. 304, 589–595 (2003).
Lucas, R., Warner, T. D., Vojnovic, I. & Mitchell, J. A. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J. 19, 635–637 (2005).
Harris, R. C. et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Invest. 94, 2504–2510 (1994).
Athirakul, K., Kim, H. S., Audoly, L. P., Smithies, O. & Coffman, T. M. Deficiency of COX-1 causes natriuresis and enhanced sensitivity to ACE inhibition. Kidney Int. 60, 2324–2329 (2001).
Epstein, M. Non-steroidal anti-inflammatory drugs and the continuum of renal dysfunction. J. Hypertens. 20 (Suppl. 6), S17–S23 (2002).
Whelton, A. COX-2-specific inhibitors and the kidney: effect on hypertension and oedema. J. Hypertens. 20 (Suppl. 6), S31–S35 (2002).
Therland, K. L. et al. Cycloxygenase-2 is expressed in vasculature of normal and ischemic adult human kidney and is colocalized with vascular prostaglandin E2 EP4 receptors. J. Am. Soc. Nephrol. 15, 1189–1198 (2004).
Singh, G. et al. Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J. Rheumatol. 30, 714–719 (2003).
Dilger, K. et al. Effects of celecoxib and diclofenac on blood pressure, renal function, and vasoactive prostanoids in young and elderly subjects. J. Clin. Pharmacol. 42, 985–994 (2002).
Schwartz, J. I. et al. Cyclooxygenase-2 inhibition by rofecoxib reverses naturally occurring fever in humans. Clin. Pharmacol. Ther. 65, 653–660 (1999).
Rossat, J., Maillard, M., Nussberger, J., Brunner, H. R. & Burnier, M. Renal effects of selective cyclooxygenase-2 inhibition in normotensive salt-depleted subjects. Clin. Pharmacol. Ther. 66, 76–84 (1999).
Patrignani, P., Capone, M. L. & Tacconelli, S. Clinical pharmacology of etoricoxib: a novel selective COX-2 inhibitor. Expert Opin. Pharmacother. 4, 265–284 (2003).
Alsalameh, S., Burian, M., Mahr, G., Woodcock, B. G. & Geisslinger, G. The pharmacological properties and clinical use of valdecoxib, a new cyclo-oxygenase-2-selective inhibitor. Aliment. Pharmacol. Ther. 17, 489–501 (2003).
Zewde, T. & Mattson, D. L. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension 44, 424–428 (2004).
Whelton, A. et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch. Intern. Med. 160, 1465–1470 (2000).
Swan, S. K. et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. A randomized, controlled trial. Ann. Intern. Med. 133, 1–9 (2000).
Whelton, A. et al. SUCCESS VI Study Group. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am. J. Ther. 8, 85–95 (2001).
Whelton, A. et al. Effects of celecoxib and rofecoxib on blood pressure and edema in patients ≥65 years of age with systemic hypertension and osteoarthritis. Am. J. Cardiol. 90, 959–963 (2002).
Mamdani, M. et al. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 363, 1751–1756 (2004).
Farkouh, M. E. et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 364, 675–684 (2004).
Day, R. Hypertension in the patient with arthritis: have we been underestimating its significance? J. Rheumatol. 30, 642–645 (2003).
Bombardier, C. et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N. Engl. J. Med. 343, 1520–1528 (2000). One of the first two large-scale clinical trials of a COX2-selective drug versus a traditional NSAID. This study generated great interest because although it showed reduced serious adverse gastrointestinal events for rofecoxib compared with naproxen it also showed increased thrombotic events.
Silverstein, F. E. et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284, 1247–1255 (2000). One of the first two large-scale clinical trials of a COX2-selective drug versus a traditional NSAID. Sparked some controversy because analysis of the full data set showed that although celecoxib produced fewer gastrointestinal adverse events than ibuprofen it was not different to diclofenac at any time point, even taking into account the consumption of aspirin.
Schnitzer, T. J. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 364, 665–674 (2004).
Topol, E. J. & Falk, G. W. A coxib a day won't keep the doctor away. Lancet 364, 639–640 (2004).
Kaufman, D. W., Kelly, J. P., Rosenberg, L., Anderson, T. E. & Mitchell, A. A. Are cyclooxygenase-2 inhibitors being taken only by those who need them? Arch. Intern. Med. 165, 1066–1067 (2005).
Graham, D. J. et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 365, 475–481 (2005).
Solomon, D. H. et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 109, 2068–2073 (2004).
Juni, P. et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 364, 2021–2029 (2004).
Bresalier, R. S. et al. Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 352, 1092–1102 (2005). Controlled trial of rofecoxib versus placebo, data from which precipitated the withdrawal of rofecoxib from the market.
Solomon, S. D. et al. Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl J Med. 352, 1071–1080 (2005).
Ott, E. et al. Efficacy and safety of the cyclo-oxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 125, 1481–1492 (2003).
Nussmeier, N. A. et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N. Engl. J. Med. 352, 1081–1091 (2005).
Reilly, I. A. & FitzGerald, G. A. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood 69, 180–186 (1987).
Capone, M. L. et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation 109, 1468–1471 (2004).
Shaya, F. T., Blume, S. W., Blanchette, C. M., Weir, M. R. & Mullins, C. D. Selective cyclooxygenase-2 inhibition and cardiovascular effects: an observational study of a Medicaid population. Arch. Intern. Med. 165, 181–186 (2005).
Johnsen, S. P. et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch. Intern. Med. 165, 978–984 (2005).
Singh, G., Mithal, A. & Triadafilopoulos, G. Both selective COX-2 inhibitors and nonselective NSAIDs increase the risk of acute myocardial infarction in patients with arthritis: selectivity is with the patient, not the drug class. Annu. Eur. Congress Rheumatol. Vienna, Austria, 8–11 June, Abstract OP0091 (2005).
Roth, S. H. Nonsteroidal antiinflammatory drug gastropathy: we started it, why don't we stop it? J. Rheumatol. 32, 1189–1191 (2005).
Olsen, N. J. Tailoring arthritis therapy in the wake of the NSAID crisis. N. Engl. J. Med. 352, 2578–2580 (2005).
Fischer, L. M., Schlienger, R. G., Matter, C. M., Jick, H. & Meier, C. R. Discontinuation of nonsteroidal anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch. Intern. Med. 164, 2472–2476 (2004).
McKeever, T. M. et al. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am. J. Respir. Crit. Care Med. 171, 966–971 (2005).
Acknowledgements
Research in T.D.W.'s laboratory is supported by the William Harvey Research Foundation and the European Community FP6 funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
J.A.M. has received consulting and/or research support from Novartis and GlaxoSmithKline. T.D.W. has received research support from AAi Pharma and Boehringer Ingelheim, and lecturing and/or consulting fees from AAi Pharma, Boehringer Ingelheim, Merck Inc., Novopharm, Pfizer and Shire Pharmaceuticals.
Related links
Glossary
- Isoforms
-
Two or more proteins having the same functions and similar (or identical) sequences that are derived from different genes.
- Peroxisome proliferator-activated receptors
-
(PPARs). A family of three nuclear receptors/transcription factors that heterodimerize with retinoid X receptors (RXR). When combined as a PPAR–RXR heterodimer, PPAR ligands and RXR ligands induce gene transcription, with selectivity being introduced through subtle differences in the 'response element' promoter regions they bind.
- Prostaglandin endoperoxides
-
PGG2 and PGH2, the first two prostanoids formed sequentially from arachidonic acid by the action of the two enzymatic sites present on COX enzymes.
- Relative risk
-
Odds of an event happening in one population relative to another. In clinical trials, often the relative risk of an adverse event happening in the test drug group is relative to a control (drug or placebo), taking the risk in the control group to be 1. Relative risk greater than 1 therefore indicates an increased risk of an event compared with control.
- Non-adjudicated investigator-reported
-
Events in a clinical trial that are reported back by the local physician (or other professional) studying the particular trial individual that are not subsequently rechecked (adjudicated) by a central review panel.
- Hazard ratio
-
Similar to relative risk. The chance of an adverse event happening in a test population compared with a control population. A hazard ratio of 2 would indicate a doubling of the chance of experiencing an adverse event.
Rights and permissions
About this article
Cite this article
Mitchell, J., Warner, T. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov 5, 75–86 (2006). https://doi.org/10.1038/nrd1929
Issue Date:
DOI: https://doi.org/10.1038/nrd1929
This article is cited by
-
Unfractionated heparin reverses aspirin inhibition of platelets during coronary artery bypass graft surgery
Scientific Reports (2024)
-
Design, synthesis, and biological evaluation of dual-target COX-2/5-LOX inhibitors for the treatment of inflammation
Medicinal Chemistry Research (2023)
-
Children with inflammatory bowel disease already have an altered arterial pulse wave
European Journal of Pediatrics (2023)
-
Microbiota-assisted therapy for systemic inflammatory arthritis: advances and mechanistic insights
Cellular and Molecular Life Sciences (2022)
-
Targeting residual inflammatory risk in coronary disease: to catch a monkey by its tail
Netherlands Heart Journal (2022)