Key Points
-
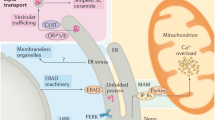
Perturbations of the endoplasmic reticulum (ER) caused by accumulation of unfolded proteins in this organelle trigger signal-transduction responses that assist with restoration of homeostasis during short-term but contribute to pathology when prolonged, including causing cell death.
-
Among the stimuli that trigger ER stress are hypoxia, oxidative injury, a high-fat diet, hypoglycaemia, protein-inclusion bodies and viral infection, thus linking these organelle-initiated responses to a diversity of diseases, including cancer, autoimmunity, diabetes, heart disease, stroke and neurodegeneration.
-
With increasing recognition of ER stress in association with human diseases and with improving understanding of the underlying molecular mechanisms, novel targets for drug discovery and new strategies for therapeutic intervention are beginning to emerge from the study of ER stress.
-
Scenarios in which ER stress contributes to disease are outlined and prospects for drug discovery are discussed.
-
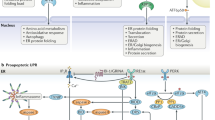
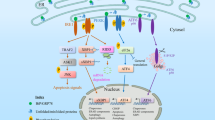
Among the cell death mechanisms addressed are: pro-apoptotic signals resulting from activation of the ER-associated kinase IRE1, an upstream activator of apoptotic signalling kinase 1 (ASK1) that activates a stress kinase pathway affecting the activity or expression of several apoptosis regulators including BCL-2, BIM and CHOP; cytoprotective ER-membrane-associated proteins that modulate ER stress signalling; and the interplay among ER-initiated signal-transduction mechanisms that control apoptosis, necrosis and autophagy.
Abstract
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) represents a cellular stress induced by multiple stimuli and pathological conditions. These include hypoxia, oxidative injury, high-fat diet, hypoglycaemia, protein inclusion bodies and viral infection. ER stress triggers an evolutionarily conserved series of signal-transduction events, which constitutes the unfolded protein response. These signalling events aim to ameliorate the accumulation of unfolded proteins in the ER; however, when these events are severe or protracted they can induce cell death. With the increasing recognition of an association between ER stress and human diseases, and with the improved understanding of the diverse underlying molecular mechanisms, novel targets for drug discovery and new strategies for therapeutic intervention are beginning to emerge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Anelli, T. & Sitia, R. Protein quality control in the early secretory pathway. EMBO J. 27, 315–327 (2008).
Pizzo, P. & Pozzan, T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 17, 511–517 (2007).
Ma, Y. & Hendershot, L. M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 28, 51–65 (2004).
Rizzuto, R., Duchen, M. R. & Pozzan, T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE 2004, re1 (2004).
Schroder, M. & Kaufman, R. J. ER stress and the unfolded protein response. Mutat. Res. 569, 29–63 (2005). An overview of the roles of ER stress and the UPR.
Rao, R. V., Ellerby, H. M. & Bredesen, D. E. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 11, 372–380 (2004).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell. Biol. 8, 519–529 (2007).
Malhotra, J. D. & Kaufman, R. J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18, 716–731 (2007).
Frand, A. R., Cuozzo, J. W. & Kaiser, C. A. Pathways for protein disulphide bond formation. Trends Cell Biol. 10, 203–210 (2000).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004). The first observation that ER-stress-induced signalling contributes to disease progression (in type 2 diabetes).
Cooper, A. A. et al. Alpha-synuclein blocks ER–Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324–328 (2006).
Delaunay, A. et al. The ER-bound ring finger protein 5 (RNF5/RMA1) causes degenerative myopathy in transgenic mice and is deregulated in inclusion body myositis. PLoS ONE 3, e1609 (2008).
Lindholm, D., Wootz, H. & Korhonen, L. ER stress and neurodegenerative diseases. Cell Death Differ. 13, 385–392 (2006).
Yoshida, H. ER stress and diseases. FEBS J. 274, 630–658 (2007). An overview of ER-stress-related diseases.
Xu, C., Bailly-Maitre, B. & Reed, J. C. Endoplamic reticulum stress: cell life and death decisions. J. Clin. Invest. 115, 2656–2664 (2005). An overview of ER stress and related cell death machinery.
Wu, J. & Kaufman, R. J. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13, 374–384 (2006).
Kaneko, M., Niinuma, Y. & Nomura, Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26, 931–935 (2003).
Egger, L. et al. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ. 10, 1188–1203 (2003).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Bernales, S., McDonald, K. L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4, e423 (2006).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Todd, D. J., Lee, A. H. & Glimcher, L. H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nature Rev. Immunol. 8, 663–674 (2008).
Rao, R. V. & Bredesen, D. E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 16, 653–662 (2004).
Kruse, K. B. et al. Mutant fibrinogen cleared from the endoplasmic reticulum via endoplasmic reticulum-associated protein degradation and autophagy: an explanation for liver disease. Am. J. Pathol. 168, 1299–1308 (2006).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Zhang, K. et al. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 115, 268–281 (2005).
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
Travers, K. J. et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 (2000).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Nishitoh, H. et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 (2002).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Hetz, C. et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312, 572–576 (2006).
Lei, K. & Davis, R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA 100, 2432–2437 (2003).
Putcha, G. V. et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38, 899–914 (2003).
Yamamoto, K., Ichijo, H. & Korsmeyer, S. J. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol. Cell. Biol. 19, 8469–8478 (1999).
Srivastava, R. K. et al. Bcl-2 and Bcl-XL block thapsigargin-induced nitric oxide generation, c-Jun NH2-terminal kinase activity, and apoptosis. Mol. Cell. Biol. 19, 5659–5674 (1999).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Fischer, H., Koenig, U., Eckhart, L. & Tschachler, E. Human caspase-12 has acquired deleterious mutations. Biochem. Biophys. Res. Comm. 293, 722–726 (2002).
Szegezdi, E., Fitzgerald, U. & Samali, A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann. NY Acad. Sci. 1010, 186–194 (2003).
Hitomi, J. et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 165, 347–356 (2004). This showed the role of CASP4 in ER-stress-induced cell death.
Salvesen, G. S. Caspases and apoptosis. Essays Biochem. 38, 9–19 (2002).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Lu, P. D., Harding, H. P. & Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27–33 (2004).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H. & Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 (2000).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005). Describes the identification of a chemical compound that inhibits ER-stress-induced cell death by modulating a UPR signal transduction event (EIF2α phosphorylation).
Kouroku, Y. et al. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 14, 230–239 (2007).
Fujita, E. et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet. 16, 618–629 (2007).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Thuerauf, D. J., Marcinko, M., Belmont, P. J. & Glembotski, C. C. Effects of the isoform-specific characteristics of ATF6α and ATF6β on endoplasmic reticulum stress response gene expression and cell viability. J. Biol. Chem. 282, 22865–22878 (2007).
Belmont, P. J. et al. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J. Biol. Chem. 283, 14012–14021 (2008).
Wang, H. G. et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284, 339–343 (1999).
Glembotski, C. C. Endoplasmic reticulum stress in the heart. Circ. Res. 101, 975–984 (2007).
Ma, Y., Brewer, J. W., Diehl, J. A. & Hendershot, L. M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 318, 1351–1365 (2002).
Oyadomari, S. & Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 (2004).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Wang, X. Z. & Ron, D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272, 1347–1349 (1996).
Bruhat, A. et al. Amino acids control mammalian gene transcription: activating transcription factor 2 is essential for the amino acid responsiveness of the CHOP promoter. Mol. Cell. Biol. 20, 7192–7204 (2000).
McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y. & Holbrook, N. J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 (2001).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R. J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 (2008).
Puthalakath, H. et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 (2007). Demonstrated a role for the BH3-only protein BIM in ER-stress-induced cell death.
Ubeda, M. et al. Stress-induced binding of the transcriptional factor CHOP to a novel DNA control element. Mol. Cell. Biol. 16, 1479–1489 (1996).
Thomenius, M. J. & Distelhorst, C. W. Bcl-2 on the endoplasmic reticulum: protecting the mitochondria from a distance. J. Cell Sci. 116, 4493–4499 (2003).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 97, 5723–5728 (2000).
Jones, R. G. et al. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca2+ homeostasis. Immunity 27, 268–280 (2007).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003).
Oakes, S. A. et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 105–110 (2005).
Chen, R. et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 (2004).
Li, C. et al. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl Acad. Sci. USA 104, 12565–12570 (2007).
White, C. et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nature Cell Biol. 7, 1021–1028 (2005).
Zhu, W., Cowie, A., Wasfy, L., Leber, B. & Andrews, D. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. EMBO J. 15, 4130–4141 (1996).
Pattingre, S. et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 122, 927–939 (2005).
Xu, Q. & Reed, J. C. BAX inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1, 337–346 (1998).
Blais, J. D. et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol. Cell. Biol. 24, 7469–7482 (2004).
Chae, H.-J. et al. Evolutionarily conserved cytoprotection provided by Bax Inhibitor-1 (BI-1) homologs from animals, plants, and yeast. Gene 323, 101–113 (2003).
Zmasek, C. M., Zhang, Q., Ye, Y. & Godzik, A. Surprising complexity of the ancestral apoptosis network. Genome Biol. 8, R226 (2007).
Chae, H. J. et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol. Cell 15, 355–366 (2004).
Xu, C., Xu, W., Palmer, A. E. & Reed, J. C. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2-family proteins. J. Biol. Chem. 283, 11477–11484 (2008).
Ng, F. W. et al. p28 Bap31, a Bcl-2/Bcl-XL-and procaspase-8-associated protein in the endoplasmic reticulum. J. Cell Biol. 139, 327–338 (1997).
Xu, K., Tavernarakis, N. & Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 31, 957–971 (2001).
Lam, D. & Golstein, P. A specific pathway inducing autophagic cell death is marked by an IP3R mutation. Autophagy 4, 349–350 (2008).
Criollo, A. et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 14, 1029–1039 (2007).
Sakaki, K., Wu, J. & Kaufman, R. J. Protein kinase Cθ is required for autophagy in response to stress in the endoplasmic reticulum. J. Biol. Chem. 283, 15370–15380 (2008).
Hoyer-Hansen, M. et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol. Cell 25, 193–205 (2007).
Unterberger, U. et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 65, 348–357 (2006).
Katayama, T. et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nature Cell Biol. 1, 479–485 (1999).
Terro, F. et al. Neurons overexpressing mutant presenilin-1 are more sensitive to apoptosis induced by endoplasmic reticulum-Golgi stress. J. Neurosci. Res. 69, 530–539 (2002).
Milhavet, O. et al. Involvement of Gadd153 in the pathogenic action of presenilin-1 mutations. J. Neurochem. 83, 673–681 (2002).
Niwa, M., Sidrauski, C., Kaufman, R. & Walter, P. A role for presenilin-1 in nuclear accumulation of ire1 fragments and induction of the mammalian unfolded protein response. Cell 99, 691–702 (1999).
Dawson, T. M. & Dawson, V. L. Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J. Clin. Invest. 111, 145–151 (2003).
Takahashi, R., Imai, Y., Hattori, N. & Mizuno, Y. Parkin and endoplasmic reticulum stress. Ann. NY Acad. Sci. 991, 101–106 (2003).
Imai, Y., Soda, M. & Takahashi, R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J. Biol. Chem. 275, 35661–35664 (2000).
Petrucelli, L. et al. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36, 1007–1019 (2002).
Ryu, E. J. et al. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J. Neurosci. 22, 10690–10698 (2002).
Paulson, H. L., Bonini, N. M. & Roth, K. A. Polyglutamine disease and neuronal cell death. Proc. Natl Acad. Sci. USA 97, 12957–12958 (2000).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008).
Kouroku, Y. et al. Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Hum. Mol. Genet. 11, 1505–1515 (2002).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Harjes, P. & Wanker, E. E. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem. Sci. 28, 425–433 (2003).
Gervais, F. G. et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nature Cell Biol. 4, 95–105 (2002).
Ybe, J. A., Mishra, S., Helms, S. & Nix, J. Crystal Structure at 2.8 Å of the DLLRKN-containing coiled-coil domain of Huntingtin-interacting protein 1 (HIP1) reveals a surface suitable for clathrin light chain binding. J. Mol. Biol. 367, 8–15 (2006).
Reed, J. C., Doctor, K. S. & Godzik, A. The domains of apoptosis: a genomics perspective. Science STKE 2004, rE9 (2004).
Roth, W. et al. Bifunctional apoptosis inhibitor (BAR) protects neurons from diverse cell death pathways. Cell Death Differ. 10, 1178–1187 (2003).
Nasir, J. et al. Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell 81, 811–823 (1995).
Kovacs, G. G. & Budka, H. Prion diseases: from protein to cell pathology. Am. J. Pathol. 172, 555–565 (2008).
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J. & Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 (2003).
Hetz, C. et al. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J. Neurosci. 25, 2793–2802 (2005).
Smith, W. W. et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant α-synuclein-induced toxicity. Hum. Mol. Genet. 14, 3801–3811 (2005).
Reijonen, S., Putkonen, N., Norremolle, A., Lindholm, D. & Korhonen, L. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp. Cell Res. 314, 950–960 (2008).
Costa-Mattioli, M. et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 (2007).
Kawaguchi, M. et al. Development of a novel fluorescent probe for fluorescence correlation spectroscopic detection of kinase inhibitors. Bioorg. Med. Chem. Lett. 18, 3752–3755 (2008).
Salh, B. c-Jun N-terminal kinases as potential therapeutic targets. Expert Opin. Ther. Targets. 11, 1339–1353 (2007).
Zhang, L. et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl Acad. Sci. USA 104, 19023–19028 (2007).
Sarkar, S. et al. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nature Chem. Biol. 3, 331–338 (2007). References 117 and 118 describe the identification of chemical compounds that induce autophagy and suppress neuronal cell death.
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Ni, M. & Lee, A. S. ER chaperones in mammalian development and human diseases. FEBS Lett. 581, 3641–3651 (2007).
Bredesen, D. E., Rao, R. V. & Mehlen, P. Cell death in the nervous system. Nature 443, 796–802 (2006).
Serradeil-Le Gal, C. et al. Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J. Clin. Invest. 98, 2729–2738 (1996).
Morello, J. P. et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Invest. 105, 887–895 (2000).
Tamarappoo, B. K. & Verkman, A. S. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J. Clin. Invest. 101, 2257–2267 (1998).
Burrows, J. A., Willis, L. K. & Perlmutter, D. H. Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α1-AT deficiency. Proc. Natl Acad. Sci. USA 97, 1796–1801 (2000).
Smith, R. A. et al. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 116, 2290–2296 (2006).
Wang, H. et al. Therapeutic gene silencing delivered by a chemically modified small interfering RNA against mutant SOD1 slows amyotrophic lateral sclerosis progression. J. Biol. Chem. 283, 15845–15852 (2008).
Uehara, T. et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006).
Yao, D. et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl Acad. Sci. USA 101, 10810–10814 (2004).
Gotoh, T. & Mori, M. Nitric oxide and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 26, 1439–1446 (2006).
Tanaka, S., Uehara, T. & Nomura, Y. Up-regulation of protein-disulfide isomerase in response to hypoxia/brain ischemia and its protective effect against apoptotic cell death. J. Biol. Chem. 275, 10388–10393 (2000).
Xu, L., Eu, J. P., Meissner, G. & Stamler, J. S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279, 234–237 (1998).
Viner, R. I., Ferrington, D. A., Williams, T. D., Bigelow, D. J. & Schoneich, C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem. J. 340, 657–669 (1999).
Xu, K. Y., Huso, D. L., Dawson, T. M., Bredt, D. S. & Becker, L. C. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc. Natl Acad. Sci. USA 96, 657–662 (1999).
Kumar, R. et al. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J. Neurochem. 77, 1418–1421 (2001).
Paschen, W., Gissel, C., Linden, T., Althausen, S. & Doutheil, J. Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res. Mol. Brain Res. 60, 115–122 (1998).
Tajiri, S. et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 11, 403–415 (2004).
Kohno, K., Higuchi, T., Ohta, S., Kumon, Y. & Sakaki, S. Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci. Lett. 224, 17–20 (1997).
Iadecola, C., Zhang, F., Casey, R., Nagayama, M. & Ross, M. E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 17, 9157–9164 (1997).
Hynes, J. Jr & Leftheri, K. Small molecule p38 inhibitors: novel structural features and advances from 2002–2005 Curr. Top. Med. Chem. 5, 967–985 (2005).
Lee, M. R. & Dominguez, C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38α protein. Curr. Med. Chem. 12, 2979–2994 (2005).
Zhai, D. et al. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J. Biol. Chem. 280, 15815–15824 (2005).
Luciano, F. et al. Cytoprotective peptide Humanin binds and inhibits pro-apoptotic Bcl-2/Bax-family protein BimEL. J. Biol. Chem. 280, 15825–15835 (2005).
Reed, J. C. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 13, 1378–1386 (2006).
Kudo, T., Imaizumi, K. & Hara, H. A molecular chaperone inducer as potential therapeutic agent for neurodegenerative disease. Nihon Shinkei Seishin Yakurigaku Zasshi 27, 63–67 (2007) (in Japanese).
Kudo, T. et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 15, 364–375 (2008). References 145 and 146 describe the identification of a cytoprotective chemical compound that upregulates a chaperone protein (GRP78).
Sokka, A. L. et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 27, 901–908 (2007).
Sekiguchi, F. et al. The potent inducible nitric oxide synthase inhibitor ONO-1714 inhibits neuronal NOS and exerts antinociception in rats. Neurosci. Lett. 365, 111–115 (2004).
Yuan, J. & Yankner, B. A. Apoptosis in the nervous system. Nature 407, 802–809 (2000).
Bossy-Wetzel, E. & Green, D. R. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem. 274, 17484–17490 (1999).
Becattini, B. et al. Structure–activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting Bid. Proc. Natl Acad. Sci. USA 103, 12602–12606 (2006).
Kakiuchi, C. et al. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nature Genet. 35, 171–175 (2003).
Cichon, S. et al. Lack of support for a genetic association of the XBP1 promoter polymorphism with bipolar disorder in probands of European origin. Nature Genet. 36, 783–784; author reply 784–785 (2004).
Kakiuchi, C. et al. Functional polymorphisms of HSPA5: possible association with bipolar disorder. Biochem. Biophys. Res. Commun. 336, 1136–1143 (2005).
Bown, C. D., Wang, J. F., Chen, B. & Young, L. T. Regulation of ER stress proteins by valproate: therapeutic implications. Bipolar Disord. 4, 145–151 (2002).
Hiroi, T., Wei, H., Hough, C., Leeds, P. & Chuang, D. M. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 5, 102–111 (2005).
Shao, L., Sun, X., Xu, L., Young, L. T. & Wang, J. F. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 78, 1317–1323 (2006).
Glembotski, C. C. The role of the unfolded protein response in the heart. J. Mol. Cell Cardiol. 44, 453–459 (2008).
Okada, K. et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110, 705–712 (2004).
Thuerauf, D. J. et al. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ. Res. 99, 275–282 (2006).
Shintani-Ishida, K., Nakajima, M., Uemura, K. & Yoshida, K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem. Biophys. Res. Commun. 345, 1600–1605 (2006).
Pan, Y. X. et al. HSP70 and GRP78 induced by endothelin-1 pretreatment enhance tolerance to hypoxia in cultured neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol. 44, S117–S120 (2004).
Vitadello, M. et al. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FESEB J. 17, 923–925 (2003).
Yamaguchi, O. et al. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc. Natl Acad. Sci. USA 100, 15883–15888 (2003).
Matsukawa, J., Matsuzawa, A., Takeda, K. & Ichijo, H. The ASK1–MAP kinase cascades in mammalian stress Response. J. Biochem. (Tokyo) 136, 261–265 (2004).
Nickson, P., Toth, A. & Erhardt, P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc. Res. 73, 48–56 (2007).
Toth, A. et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia–reperfusion. Am. J. Physiol. Heart Circ. Physiol. 291, H52–H60 (2006).
Ramadan, S. et al. p73 induces apoptosis by different mechanisms. Biochem. Biophys. Res. Commun. 331, 713–717 (2005).
Terrinoni, A. et al. p73-α is capable of inducing scotin and ER stress. Oncogene 23, 3721–3725 (2004).
Melino, G. et al. p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J. Biol. Chem. 279, 8076–8083 (2004).
Martindale, J. J. et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 98, 1186–1193 (2006).
Severino, A. et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 50, 1029–1037 (2007).
Gargalovic, P. S. et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 2490–2496 (2006).
Li, Y. et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and MAP kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 280, 21763–21772 (2005).
Zhang, C. et al. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem. Biophys. Res. Commun. 289, 718–724 (2001).
Lee, A. H., Scapa, E. F., Cohen, D. E. & Glimcher, L. H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320, 1492–1496 (2008).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Araki, E., Oyadomari, S. & Mori, M. Endoplasmic reticulum stress and diabetes mellitus. Intern. Med. 42, 7–14 (2003).
Fonseca, S. G. et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic β-cells. J. Biol. Chem. 280, 39609–39615 (2005).
Yamada, T. et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum. Mol. Genet. 15, 1600–1609 (2006).
Ishihara, H. et al. Disruption of the WFS1 gene in mice causes progressive β-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum. Mol. Genet. 13, 1159–1170 (2004).
Oyadomari, S. et al. Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc. Natl Acad. Sci. USA 98, 10845–10850 (2001).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Cnop, M. et al. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic β-cell dysfunction and apoptosis. J. Biol. Chem. 282, 3989–3997 (2007).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006). Describes the use of a chemical chaperone to reduce ER stress for treatment of type 2 diabetes in rodents.
Jeffrey, K. D. et al. Carboxypeptidase E mediates palmitate-induced β-cell ER stress and apoptosis. Proc. Natl Acad. Sci. USA 105, 8452–8457 (2008).
Shuda, M. et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 38, 605–614 (2003).
Fujimoto, T. et al. Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer 10, 301–306 (2003).
Jamora, C., Dennert, G. & Lee, A. S. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA 93, 7690–7694 (1996).
Romero-Ramirez, L. et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 64, 5943–5947 (2004).
Bi, M. et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 24, 3470–3481 (2005).
Park, H. R. et al. Effect on tumor cells of blocking survival response to glucose deprivation. J. Natl Cancer Inst. 96, 1300–1310 (2004).
Lee, A. S. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 67, 3496–3499 (2007).
Dent, P., Yacoub, A., Grant, S., Curiel, D. T. & Fisher, P. B. MDA-7/IL-24 regulates proliferation, invasion and tumor cell radiosensitivity: a new cancer therapy? J. Cell Biochem. 95, 712–719 (2005).
Lee, A. H., Iwakoshi, N. N., Anderson, K. C. & Glimcher, L. H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA 100, 9946–9951 (2003).
Nawrocki, S. T. et al. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res. 65, 11510–11519 (2005).
Nawrocki, S. T. et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 65, 11658–11666 (2005).
Pyrko, P. et al. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res. 67, 10920–10928 (2007).
Gills, J. J. et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin. Cancer Res. 13, 5183–5194 (2007).
Phillips, L. R., Wolfe, T. L., Malspeis, L. & Supko, J. G. Analysis of brefeldin A and the prodrug breflate in plasma by gas chromatography with mass selective detection. J. Pharm. Biomed. Anal. 16, 1301–1309 (1998).
Carew, J. S. et al. Targeting endoplasmic reticulum protein transport: a novel strategy to kill malignant B cells and overcome fludarabine resistance in CLL. Blood 107, 222–231 (2006).
Reimold, A. M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Neubert, K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nature Med. 14, 748–755 (2008).
Iwakoshi, N. N., Pypaert, M. & Glimcher, L. H. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275 (2007).
Paton, A. W. et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443, 548–552 (2006).
Nagaraju, K. et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 (2005).
Blass, S. et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 44, 761–771 (2001).
Corrigall, V. M. et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J. Immunol. 166, 1492–1498 (2001).
Lin, W. et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 117, 448–456 (2007).
Ito, Y. et al. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 21, 6233–6242 (2001).
Bourdon, J. C., Renzing, J., Robertson, P. L., Fernandes, K. N. & Lane, D. P. Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J. Cell Biol. 158, 235–246 (2002).
Lotz, K., Pyrowolakis, G. & Jentsch, S. BRUCE, a giant E2/E3 ubiquitin ligase and inhibitor of apoptosis protein of the trans-Golgi network, is required for normal placenta development and mouse survival. Mol. Cell. Biol. 24, 9339–9350 (2004).
Hao, Y. et al. Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nature Cell Biol. 6, 849–860 (2004).
Qiu, X. B. & Goldberg, A. L. The membrane-associated inhibitor of apoptosis protein, BRUCE/Apollon, antagonizes both the precursor and mature forms of Smac and caspase-9. J. Biol. Chem. 280, 174–182 (2005).
Sekine, K. et al. HtrA2 cleaves Apollon and induces cell death by IAP-binding motif in Apollon-deficient cells. Biochem. Biophys. Res. Commun. 330, 279–285 (2005).
Tenev, T., Zachariou, A., Wilson, R., Paul, A. & Meier, P. Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1. EMBO J. 21, 5118–5129 (2002).
Kang, K. W., Lee, S. J. & Kim, S. G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 7, 1664–1673 (2005).
Satoh, T. et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc. Natl Acad. Sci. USA 103, 768–773 (2006).
Kraft, A. D., Johnson, D. A. & Johnson, J. A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 24, 1101–1112 (2004).
Shih, A. Y., Li, P. & Murphy, T. H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 25, 10321–10335 (2005).
Dinkova-Kostova, A. T. et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc. Natl Acad. Sci. USA 102, 4584–4589 (2005).
Satoh, T. et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S.-alkylation of targeted cysteines on Keap1. J. Neurochem. 104, 1116–1131 (2008).
Lee, G. H. et al. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J. Biol. Chem. 282, 21618–21628 (2007).
Szabadkai, G. et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 (2004).
Lutter, M. et al. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nature Cell Biol. 2, 754–756 (2000).
Kuwana, T. et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 (2002).
Benhar, M., Forrester, M. T. & Stamler, J. S. Nitrosative stress in the ER: a new role for S-nitrosylation in neurodegenerative diseases. ACS Chem. Biol. 1, 355–358 (2006).
Wood, D. E. et al. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17, 1069–1078 (1998).
Chen, M. et al. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J. Biol. Chem. 276, 30724–30728 (2001).
Yousefi, S. et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature Cell Biol. 8, 1124–1132 (2006).
Bernardi, P. et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 273, 2077–2099 (2006).
Wang, J. F., Bown, C. & Young, L. T. Differential display PCR reveals novel targets for the mood-stabilizing drug valproate including the molecular chaperone GRP78. Mol. Pharmacol. 55, 521–527 (1999).
Youn, H., Sun, L., Prywes, R. & Liu, J. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor Mef2. Science 286, 790–793 (1999).
Edlich, F. et al. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. EMBO J. 24, 2688–2699 (2005).
Liu, H., Peng, H. W., Cheng, Y. S., Yuan, H. S. & Yang-Yen, H. F. Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol. Cell. Biol. 25, 3117–3126 (2005).
Shohat, G., Shani, G., Eisenstein, M. & Kimchi, A. The DAP-kinase family of proteins: study of a novel group of calcium-regulated death-promoting kinases. Biochim. Biophys. Acta 1600, 45–50 (2002).
Bialik, S. & Kimchi, A. DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin. Cancer Biol. 14, 283–294 (2004).
Uchikawa, O., Sakai, N., Terao, Y. & Suzuki, H. Fused heterocyclic compound. WO2008016131 (2008).
Acknowledgements
We thank T. Siegfried for manuscript preparation, and the National Institutes of Health for generous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.C.R. has served on the board of ISIS Pharmaceuticals.
Related links
Glossary
- Molecular chaperone
-
A molecular chaperone is a protein that aids the folding of other proteins. Some molecular chaperones reside in the lumen of the endoplasmic reticulum, such as GRP78, a member of the HSP70 family, and GRP94, a member of the HSP90 family.
- Protein disulphide isomerase
-
(PDI). A cellular enzyme in the lumen of the endoplasmic reticulum of eukaryotes or the periplasmic region of prokaryotes. This enzyme catalyses the formation and breakage of disulphide bonds between cysteine residues in proteins, which affects protein folding.
- Unfolded protein response
-
(UPR). A conserved physiological response involving endoplasmic reticulum (ER)-initiated signal-transduction events, induced by accumulation of unfolded proteins in the lumen of the ER. In mammals, the UPR includes signals initiated by ER membrane-associated proteins: IRE1, PERK and ATF6.
- ER stress
-
An organelle-initiated cell stress condition, typically associated with accumulation of misfolded or unfolded proteins in the lumen of the ER. ER stress is caused by a wide diversity of stimuli.
- ER-assisted degradation
-
(ERAD). ERAD involves the retrograde translocation of unfolded proteins from the lumen of the ER to the cytosol, where ER membrane-associated ubiquitin ligases post-translationally modify the translocated proteins thereby targeting them for degradation, usually by the 26S proteasome.
- Autophagy
-
Autophagy, or autophagocytosis, is a catabolic celullar process involving the lysosome-dependent degradation of macromolecules, organelles and other cell components. Autophagy plays housekeeping roles in protein degradation, complementing the proteasome-based protein degradation system. Autophagy can also be important for cell survival during times of nutrient deprivation and hypoxia, and is induced in some cases by endoplasmic reticulum stress. Autophagy has also been associated with cell death in some contexts.
- EIF2α
-
(Eukaryotic translation initiation factor 2α). The translation initiation complex EIF2 is a heterotrimer of EIF2α, EIF2β and EIF2γ. This complex binds to GTP and Met-tRNA. It transfers Met-tRNA to the 40S subunit of the ribosome to form the 43S pre-initiation complex. Successive rounds of translation and initiation are promoted by exchanging GDP for GTP. Phosphorylation of EIF2α by PERK inactivates EIF2α, resulting in inhibition of cap-dependent translation initiation.
- S-nitrosylation
-
S-nitrosylation describes the covalent attachment of a nitrogen monoxide group to the thiol (-SH) of cysteines in proteins. It is a post-translational modification of proteins that can modulate cellular signalling, which provides a mechanism for redox-based physiological regulation.
Rights and permissions
About this article
Cite this article
Kim, I., Xu, W. & Reed, J. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7, 1013–1030 (2008). https://doi.org/10.1038/nrd2755
Issue Date:
DOI: https://doi.org/10.1038/nrd2755
This article is cited by
-
Therapeutic potential of berberine in attenuating cholestatic liver injury: insights from a PSC mouse model
Cell & Bioscience (2024)
-
Combined proteomic and metabolomic studies on the liver of Amur sturgeon Acipenser schrenckii under titanium dioxide nanoparticle exposure
Journal of Oceanology and Limnology (2024)
-
Mitochondrial dysfunction at the crossroad of cardiovascular diseases and cancer
Journal of Translational Medicine (2023)
-
PMCA inhibition reverses drug resistance in clinically refractory cancer patient-derived models
BMC Medicine (2023)
-
Adenosine 2 receptor regulates autophagy and apoptosis to alleviate ischemia reperfusion injury in type 2 diabetes via IRE-1 signaling
BMC Cardiovascular Disorders (2023)