Key Points
-
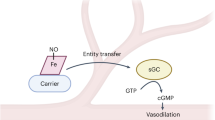
Nitric oxide (NO) is an intracellular and intercellular messenger molecule that has been implicated in a wide range of physiological processes.
-
Overproduction of NO can cause tissue damage and dysregulated cellular signalling, and seems to be a key feature of pathogenesis in several inflammatory and degenerative disorders.
-
Three isoforms of nitric oxide synthase (NOS) have been identified: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). Although all three have important physiological roles, it is overproduction of NO from iNOS and nNOS that is important in certain disease states. These isoforms are therefore potential targets for new treatments. NO donation or boosting NO effects is also a target for treatments, but this article focuses solely on inhibition.
-
Studies with pharmacological inhibitors of NOS isozymes, and NOS-isoform knockout mice, indicate that blockade of individual NOS isozymes might produce therapeutic benefit, but will also be associated with harmful effects. For long-term treatment, a drug must not inhibit eNOS, as this would cause atherosclerosis and have significant adverse cardiovascular effects.
-
The active site of NOS isozymes is significantly conserved, so that isoform selectivity has not been easy to achieve. Nonetheless, compounds are now available that show specificity for inhibition of iNOS or nNOS over eNOS. Furthermore, compounds have been identified that inhibit activity through inhibition of dimerization or inhibition of cofactor binding. Compounds that block NO generation have therapeutic potential, and some are close to entering clinical trials. Potential targets include asthma, arthritis, neurodegenerative disorders, cancer, gastrointestinal inflammation and pain.
-
Even isoform-selective inhibition of NOS is likely to be associated with significant unwanted side effects. Partial inhibition of NOS isozymes might be a better option than complete or near complete inhibition. Even then, it seems that the role of NO might change from harmful to helpful during the course of a single process. For example, iNOS activity can be involved in tissue destruction, and in wound repair and resolution of inflammation.
-
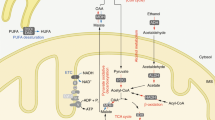
Alternative approaches to decreasing harmful generation of NO while leaving physiological NO unaffected include inhibition of enzymes that regulate cofactors for NOS, manipulation of substrate levels or inhibition of enzymes that metabolize endogenous inhibitors of NOS.
-
Drugs that affect the activity of specific NOS isoforms are soon to enter clinical trials. Earlier experimental studies in humans with isoform non-selective inhibitors indicate that major biological effects can be expected, and not all of them will be beneficial. Nonetheless, the potential for therapeutic benefit makes it important that such drugs enter trials and are evaluated in clinical disease.
Abstract
Nitric oxide (NO) is a key physiological mediator, and the association of disordered NO generation with many pathological conditions has led to much interest in pharmacologically modulating NO levels. However, the wide range of processes in which NO has been implicated, and the fact that increases or decreases in NO levels might be therapeutically desirable depending on the condition or even at different stages of the same condition, pose considerable challenges for drug development. Here, we focus on the rationale and potential for approaches that reduce NO synthesis, which have led to the development of several compounds that will shortly be entering clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Hanafy, K. A., Krumenacker, J. S. & Murad, F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med. Sci. Monit. 7, 801–819 (2001).
Ignarro, L. J., Napoli, C. & Loscalzo, J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide. Circ. Res. 90, 21–28 (2002).
Alderton, W. K., Cooper, C. E. & Knowles, R. G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357, 593–615 (2001). A comprehensive review of the biochemistry and some structural aspects of NOSs.
Cabe, T. J., Fulton, D., Roman, L. J. & Sessa, W. C. Enhanced electron flux and reduced calmodulin dissociation may explain 'calcium-independent' eNOS activation by phosphorylation. J. Biol. Chem. 275, 6123–6128 (2000).
Lowenstein, C. J. Purification and assessment of proteins associated with nitric oxide synthase. Methods Enzymol. 353, 233–240 (2002).
Ignarro, L. J., Cirino, G., Casini, A. & Napoli, C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 34, 879–886 (1999).
Davis, K. L., Martin, E., Turko, I. V. & Murad, F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 41, 203–236 (2001).
Ischiropoulos, H. & Gow, A. J. Nitric oxide chemistry and cellular signaling. J. Cell. Physiol. 187, 277–282 (2001).
O'Donnell, V. B. et al. Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods Enzymol. 301, 454–470 (1999).
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation, the prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001). The recognition that NO might affect many proteins through nitrosation reactions increases the range of effects of NO and the possibility that these will vary with the redox state of the cell. This clearly has implications for understanding the long-term effects of inhibitors.
Brown, G. C. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome coxidase. Biochim. Biophys. Acta 1504, 46–57 (2001).
Napoli, C. & Ignarro, L. J. Nitric oxide and atherosclerosis. Nitric Oxide 5, 88–97 (2001).
Cayatte, A. J., Palacino, J. J., Horten, K. & Cohen, R. A. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler. Thromb. 14, 753–759 (1994).
Kuhlencordt, P. J. et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischaemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104, 448–454 (2001).
Rand, M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin. Exp. Pharmacol. Physiol. 19, 147–169 (1992).
Baranano, D. E. & Snyder, S. H. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc. Natl Acad. Sci. USA 98, 10996–11002 (2001).
Burnett, A. L. et al. Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nature Med. 3, 571–574 (1997).
Burnett, A. L. et al. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 2, 288–296 (1996).
Gyurko, R., Leupen, S. & Huang, P. L. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143, 2767–2774 (2002).
Kobzik, L., Reid, M. B., Bredt, D. S. & Stamler, J. S. Nitric oxide in skeletal muscle. Nature 8, 546–548 (1994).
Brenman, J. E., Chao, D. S., Xia, H., Aldape, K. & Bredt, D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 8, 743–752 (1995).
Barouch, L. A. et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416, 337–339 (2002).
De Sanctis, G. T. et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J. Exp. Med. 189, 1621–1629 (1999).
Moroi, M. et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J. Clin. Invest. 101, 1225–1232 (1998).
Hibbs, J. B. Jr, Taintor, R. R., Vavrin, Z. & Rachlin, E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157, 87–94 (1988).
Marletta, M. A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell 78, 927–930 (1994).
Vallance, P. & Charles, I. Nitric oxide in sepsis: of mice and men. Sepsis 1, 93–100 (1998).
Bogdan, C. Nitric oxide and the immune response. Nature Immunol. 2, 907–916 (2001). The pleiotropic effects of NO are nowhere more evident than in the immune system. This review outlines these effects (some of which seem to oppose each other), describes some of the controversy about iNOS in humans and discusses the problems that face NOS inhibitors.
Niedbala, W., Wei, X. Q., Piedrafita, D., Xu, D. & Liew, F. Y. Effects of nitric oxide on the induction and differentiation of TH1 cells. Eur. J. Immunol. 29, 2498–2505 (1999).
van't Hof, R. J. et al. Requirement of the inducible nitric oxide synthase pathway for IL-1-induced osteoclastic bone resorption. Proc. Natl Acad. Sci. USA 97, 7993–7998 (2000).
Kenyon, N. J. et al. Susceptibility to ozone-induced acute lung injury in iNOS-deficient mice. Am. J. Physiol. 282, L540–L545 (2002).
Arnett, H. A. et al. The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. J. Immunol. 168, 427–433 (2002).
Kane, A. J. et al. Inducible nitric oxide synthase (iNOS) activity promotes ischaemic skin flap survival. Br. J. Pharmacol. 132, 1631–1638 (2001).
Nandagopal, K., Dawson, T. M. & Dawson, V. L. Critical role for nitric oxide signaling in cardiac and neuronal ischaemic preconditioning and tolerance. J. Pharmacol. Exp Ther. 297, 474–478 (2002).
Luo, Z. D. & Cizkova, D. The role of nitric oxide in nociception. Curr. Rev. Pain 4, 459–466 (2000).
Saban, M. R., Nguyen, N. B., Hammond, T. G. & Saban, R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am. J. Pathol. 160, 2095–2110 (2002).
van't Hof, R. J. & Ralston, S. H. Nitric oxide and bone. Immunology 103, 255–261 (2001).
Kubes, P. & McCafferty, D. M. Nitric oxide and intestinal inflammation. Am. J. Med. 109, 150–158 (2000).
Sanders, S. P. Nitric oxide in asthma: pathogenic, therapeutic or diagnostic? Am. J. Respir. Cell Mol. Biol. 21, 147–149 (1999).
Silkoff, P. E., Robbins, R. A., Gaston, B., Lundberg, J. O. & Townley, R. G. Endogenous nitric oxide in allergic airway disease. J. Allergy Clin. Immunol. 105, 438–448 (2000).
Iravani, M. M., Kashefi, K., Mander, P., Rose, S. & Jenner, P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience 110, 49–58 (2002).
Wada, K., Chatzipanteli, K., Kraydieh, S., Bustol, R. & Dietrich, W. D. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery 43, 1427–1436 (1998).
Heneka, M. T. & Feinstein, D. L. Expression and function of inducible nitric oxide synthase in neurons. J. Neuroimmunol. 114, 8–18 (2001).
Izumi, Y., Benz, A. M., Clifford, D. B. & Zorumski, C. F. Nitric oxide inhibitors attenuate N-methyl-D-aspartate excitotoxicity in rat hippocampal slices. Neurosci. Lett. 135, 227–230 (1992).
Huang, Z. et al. Effects of cerebral ischaemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 (1994). The original paper that identified the neurotoxic effects of nNOS and proposed a protective role for eNOS in stroke.
Ando, A. et al. Nitric oxide is proangiogenic in the retina and choroid. J. Cell. Physiol. 191, 116–124 (2002).
Bhagat, K., Hingorani, A. D., Palacios, M., Charles, I. G. & Vallance P. Cytokine-induced venodilatation in humans in vivo: eNOS masquerading as iNOS. Cardiovasc. Res. 41, 754–764 (1999).
Haendeler, J. et al. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nature Cell Biol. 4, 743–749 (2002).
Moncada, S. & Erusalimsky, J. D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nature Rev. Mol. Cell Biol. 3, 214–220 (2002).
Lala, P. K. & Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2, 149–156 (2001).
Huang, P. L. Mouse models of nitric oxide synthase deficiency. J. Am. Soc. Nephrol. 11, S120–S123 (2000).
Dawson, V. L. & Dawson, T. M. Nitric oxide in neurodegeneration. Prog. Brain Res. 118, 215–229 (1998).
Dawn, B. & Bolli, R. Role of nitric oxide in myocardial preconditioning. Ann. NY Acad. Sci. 962, 18–41 (2002).
Kim, P. K., Kwon, Y. G., Chung, H. T. & Kim, Y. M. Ann. NY Acad. Sci. 962, 42–52 (2002).
Kubes, P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut 47, 6–9 (2000).
Corbett, J. A. & McDaniel, M. L. The use of aminoguanidine, a selective iNOS inhibitor, to evaluate the role of nitric oxide in the development of autoimmune diabetes. Methods 10, 21–30 (1996).
Stamler, J. S., Loh, E., Roddy, M. A., Currie, K. E. & Creager, M. A. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 89, 2035–2040 (1994).
Petros, A., Bennett, D. & Vallance, P. Effects of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet 338, 1557–1558 (1991). The first administration of an NOS inhibitor to patients. This paper shows that the haemodynamic effects of septic shock can be reversed by blocking NOS.
Petros, A. et al. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc. Res. 28, 34–39 (1994).
Ashina, M., Lassen, L. H., Bendtsen, L., Jensen, R. & Olesen, J. Effect of inhibition of nitric oxide synthase on chronic tension-type headache: a randomised crossover trial. Lancet 353, 287–289 (1999).
Warren, J. B. Nitric oxide and human skin blood flow responses to acetylcholine and ultraviolet light. FASEB J. 8, 247–251 (1994).
Rees, D. D., Palmer, R. M., Schulz, R., Hodson, H. F. & Moncada, S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 101, 746–752 (1990).
Garvey, E. P. et al. Potent and selective inhibition of human nitric oxide synthases. Inhibition of non-amino acid isothioureas. J. Biol. Chem. 269, 26669–26676 (1994).
Raman, C. S. et al. Implications for isoform-selective inhibitor design derived from the binding mode of bulky isothioureas to the heme domain of endothelial nitric oxide synthase. J. Biol. Chem. 276, 26486–26491 (2001). A structural analysis of the binding of NOS inhibitors. This paper begins to define some of the potential structural requirements for isoform-selective inhibition of NOS.
Li, H. et al. Crystal structures of zinc-free and bound heme domain of human inducible nitric oxide synthase: implications for dimer stability and comparison with endothelial nitric oxide synthase. J. Biol. Chem. 274, 21276–21284 (1999).
Garvey, E. P. et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. J. Biol. Chem. 272, 4959–4963 (1997).
Evans, S. M. & Whittle, B. J. Interactive roles of superoxide and inducible nitric oxide synthase in rat intestinal injury provoked by non-steroidal anti-inflammatory drugs. Eur. J. Pharmacol. 429, 287–296 (2001).
Beaton, H., Hamley, P., Nicholls, D. J., Tinker, A. C. & Wallace, A. V. 3,4-Dihydro-1-isoquinolinamines: a novel class of nitric oxide synthase inhibitors with a range of isoform selectivity and potency. Biorganic Med. Chem. Lett. 11, 1023–1026 (2001). This paper describes a new class of selective iNOS inhibitors. A member of this class is apparently close to entering clinical trials.
Moore, W. M. et al. L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J. Med. Chem. 37, 3886–3888 (1994).
Moore, W. M. et al. Synthesis and biological characterization of L-N6-(1-iminoethyl)lysine 5-tetrazole-amide, a prodrug of a selective iNOS inhibitor. J. Med. Chem. 45, 1686–1689 (2002).
McMillan, K. et al. Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry. Proc. Natl Acad. Sci. USA 97, 1506–1511 (2000).
Blasko, E. et al. Mechanistic studies with potent and selective inducible nitric oxide synthase dimerization inhibitors. J. Biol. Chem. 277, 295–302 (2002).
Wolff, D. J., Datto, G. A. & Samatovicz, R. A. The dual mode of inhibition of calmodulin-dependent nitric oxide synthase by antifungal imidazole agents. J. Biol. Chem. 268, 9430–9436 (1993).
Bogle, R. G. & Vallance, P. Functional effects of econazole on inducible nitric oxide synthase: production of a calmodulin-dependent enzyme. Br. J. Pharmacol. 117, 1053–1058 (1996).
Matter, H. et al. Structural requirements for inhibition of the neuronal nitric oxide synthase (NOS-1): 3D-QSAR analysis of 4-oxo and 4-amino-pteridine-based inhibitors. J. Med. Chem. 45, 2923–2941 (2002).
Jaffrey, S. R. & Snyder, S. H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science 274, 774–777 (1996).
Huang, H., Martasek, P., Roman, L. J. & Silverman, R. B. Synthesis and evaluation of peptidomimetics as selective inhibitors and active site probes of nitric oxide synthases. J. Med. Chem. 43, 2938–2945 (2000).
O'Neill, M. J. et al. ARL 17477, a selective nitric oxide synthase inhibitor, with neuroprotective effects in animal models of global and focal cerebral ischaemia. Brain Res. 871, 234–244 (2000).
Babu, B. R. & Griffith, O. W. N5-(1-imino-3-butenyl)-L-ornithine, a neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J. Biol. Chem. 273, 8882–8889 (1998).
Radomski, M. W., Palmer, R. M. & Moncada, S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc. Natl Acad. Sci. USA 87, 10043–10047 (1990).
Vallance, P., Leone, A., Calver, A., Collier, J. & Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339, 572–575 (1992).
Leiper, J. & Vallance, P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 43, 542–548 (1999).
Ogawa, T., Kimoto, M. & Sasaoka, K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem. 264, 10205–10209 (1989).
Leiper, J. M. et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem. J. 343, 209–214 (1999)
MacAllister, R. J. et al. Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. Br. J. Pharmacol. 119, 1533–1540 (1996).
Nakagomi, S., Kiryu-Seo, S., Kimoto, M., Emson, P. C. & Kiyama, H. Dimethylarginine dimethylaminohydrolase (DDAH) as a nerve-injury-associated molecule: mRNA localization in the rat brain and its coincident up-regulation with neuronal NO synthase (nNOS) in axotomized motoneurons. Eur. J. Neurosci. 11, 2160–2166 (1999).
Abe, T., Toghi, H., Murata, T., Isobe, C. & Sato, C. Reduction is asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in the cerebrospinal fluid during aging and in patients with Alzheimer's disease. Neurosci. Lett. 312, 177–179 (2001).
Murray-Rust, J. et al. Structural insights into the hydrolysis of cellular nitric oxide synthase inhibitors by dimethylarginine dimethylaminohydrolase. Nature Struct. Biol. 8, 679–683 (2001). Blocking DDAH leads to an accumulation of naturally occurring NOS inhibitors. This paper provides structural insight into enzyme function and possibilities for inhibition of DDAH as a route to affect NO generation.
Xie, L., Hattori, Y., Tume, N. & Gross, S. S. The preferred source of arginine for high-output nitric oxide synthesis in blood vessels. Semin. Perinatol. 24, 42–45 (2000).
Iwata, S. et al. Endothelial nitric oxide synthase expression in tumor vasculature is correlated with malignancy in human supratentorial astrocytic tumors. Neurosurgery 45, 24–28 (1999).
Leiper, J., Murray-Rust, J., MacDonald, N. & Vallance, P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and ADMA pathways. Proc. Natl Acad. Sci. USA 99, 13527–13532 (2002).
Drapier, J. C. & Hibbs, J. B. Jr. Aconitases: a class of metalloproteins highly sensitive to nitric oxide synthesis. Methods Enzymol. 269, 26–36 (1996).
Sun, J., Xin, C., Eu, J. P., Stamler, J. S. & Meissner, G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl Acad. Sci. USA 98, 11158–11162 (2001).
Guittet, O. et al. Differential sensitivity of the tyrosyl radical of mouse ribonucleotide reductase to nitric oxide and peroxynitrite. J. Biol. Chem. 273, 22136–22144 (1998).
Goodwin, D. C. et al. Nitric oxide trapping of tyrosyl radicals generated during prostaglandin endoperoxide synthase turnover. Detection of the radical derivative of tyrosine 385. J. Biol. Chem. 273, 8903–8909 (1998).
Huang, P. L. et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995).
Lefer, D. J. et al. Leukocyte–endothelial cell interactions in nitric oxide synthase-deficient mice. Am. J. Physiol. 276, 1943–1950 (1999).
Gyurko, R., Kuhlencordt, P., Fishman, M. C. & Huang, P. L. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am. J. Physiol. 278, H971–H981 (2000).
Huang, Z. et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J. Cereb. Blood Flow Metab. 16, 981–987 (1996).
Lo, E. H. et al. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke 27, 1381–1385 (1996).
Kano, T., Shimizu-Sasamata, M., Huang, P. L., Moskowitz, M. A. & Lo, E. H. Effects of nitric oxide synthase gene knockout on neurotransmitter release in vivo. Neuroscience 86, 695–699 (1998).
Panahian, N. et al. Attenuated hippocampal damage after global cerebral ischemia in mice mutant in neuronal nitric oxide synthase. Neuroscience 72, 343–354 (1996).
Kakuyama, M., Ahluwalia, A., Rodrigo, J. & Vallance, P. Cholinergic contraction is altered in nNOS knockouts: co-operative modulation of neural bronchoconstriction by nNOS and COX. Am. J. Respir. Crit. Care Med. 160, 2072–2078 (1999).
Huang, P. L., Dawson, T. M., Bredt, D. S., Snyder, S. H. & Fishman, M. C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273–1286 (1993). The original paper that describes the striking gastrointestinal phenotype of nNos knockout mice.
Mashimoto, H., He, X. D., Huang, P. L., Fishman, M. C. & Goyal, R. K. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J. Clin. Invest. 98, 8–13 (1996).
MacMicking, J. D. et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 541–650 (1995).
Wei, X. Q. et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375, 408–411 (1995). References 106 and 107 describe the effects of loss of iNOS. There are some interesting differences between the phenotypes described.
Gross, S. S., Kilbourn, R. G. & Griffith, O. W. NO in septic shock: good, bad or ugly? Learning from iNOS knockouts. Trends Microbiol. 4, 47–49 (1996).
Scott, D. J. et al. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the Apc(Min/+) mouse. Gastroenterology 121, 889–899 (2001).
Yamasaki, K. et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J. Clin. Invest. 101, 967–971 (1998).
Heneka, M. T. et al. Neuronal and glial coexpression of argininosuccinate synthetase and inducible nitric oxide synthase in Alzheimer disease. J. Neuropathol. Exp. Neurol. 60, 906–916 (2001).
Easki, T. et al. Expression of inducible nitric oxide synthase and Fas/Fas ligand correlates with the incidence of apoptotic cell death in atheromatous plaques of human coronary arteries. Nitric Oxide 4, 561–571 (2000).
Behr-Roussel, D., Rupin, A., Sansilvestri-Morel, P., Fabiani, J. N. & Verbeuren, T. J. Histochemical evidence for inducible nitric oxide synthase in advanced but non-ruptured human atherosclerotic carotid arteries. Histochem. J. 32, 41–51 (2000).
Murray, I. A., Daniels, I., Coupland, K., Smith, J. A. & Long, R. G. Increased activity and expression of iNOS in human duodenal enterocytes from patients with celiac disease. Am. J. Physiol. Gastrointest. Liver. Physiol. 283, 19–26 (2002).
Dijkstra, G. et al. Increased expression of inducible nitric oxide synthase in circulating monocytes from patients with active inflammatory bowel disease. Scand. J. Gastroenterol. 37, 546–554 (2002).
Perner, A. et al. Expression of nitric oxide synthases and effects of L-arginine and L-NMMA on nitric oxide production and fluid transport in collagenous colitis. Gut 49, 387–394 (2001).
Liu, J. S., Zhao, M. L., Brosnan, C. F. & Lee, S. C. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am. J. Pathol. 158, 2057–2066 (2001).
Vuolteenaho, K., Moilanen, T., Al-Saffar, N., Knowles, R. G. & Moilanen, E. Regulation of the nitric oxide production resulting from the glucocorticoid-insensitive expression of iNOS in human osteoarthritic cartilage. Osteoarthritis Cartilage 9, 597–605 (2001).
Borderie, D. et al. Nitric oxide synthase is expressed in the lymphomononuclear cells of synovial fluid in patients with rheumatoid arthritis. J. Rheumatol. 26, 2083–2088 (1999).
Knott, C., Stern, G. & Wilkin, G. P. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and-2. Mol. Cell. Neurosci. 16, 724–39 (2000).
Annane, D. et al. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet 355, 1143–1148 (2001).
Forster, C., Clark, H. B., Ross, M. E. & Iadecola, C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta. Neuropathol. 97, 215–220 (1999).
Suda, O. et al. Long-term treatment with Nω-nitro-L-arginine methyl ester (L-NAME) causes arteriosclerotic coronary lesions in endothelial nitric oxide synthase-deficient mice. Circulation 106, 1729–1735 (2002).
Acknowledgements
Work in the laboratory of P. V. is generously supported by grants from the British Heart Foundation, the Wellcome Trust and the Medical Research Council. The authors are grateful to R. Knowles, N. Boughton-Smith, and C. S. Raman for information provided, and to A. Hobbs for critical reading of the manuscript. Figure 3 was prepared by S. Rossiter. From the work of the authors, University College London (UCL) holds patents in relation to DDAH as a target for drug action.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
arginine glycine amidinotransferase
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
nitric oxide: role in human disease
Glossary
- APOPTOSIS
-
Programmed cell death.
- CASPASES
-
A family of intracellular cysteine proteases that are responsible for apoptosis.
- PRODRUG
-
A pharmacologically inactive compound that is converted to the active form of the drug by endogenous enzymes or metabolism. It is generally designed to overcome problems associated with stability, toxicity, lack of specificity or limited (oral) bioavailability.
Rights and permissions
About this article
Cite this article
Vallance, P., Leiper, J. Blocking NO synthesis: how, where and why?. Nat Rev Drug Discov 1, 939–950 (2002). https://doi.org/10.1038/nrd960
Issue Date:
DOI: https://doi.org/10.1038/nrd960
This article is cited by
-
DDAH-1, via regulation of ADMA levels, protects against ischemia-induced blood-brain barrier leakage
Laboratory Investigation (2021)
-
Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism
Nature Methods (2017)
-
Synthesis of oxadiazoline and quinazolinone derivatives and their biological evaluation as nitric oxide synthase inhibitors
Medicinal Chemistry Research (2016)
-
The Effects of Dexamethasone and L-NAME on Acute Lung Injury in Rats with Lung Contusion
Inflammation (2016)
-
Levosimendan affects oxidative and inflammatory pathways in the diaphragm of ventilated endotoxemic mice
Critical Care (2015)