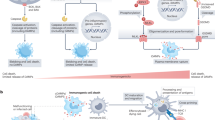
Key Points
-
Three p38 mitogen-activated protein kinase (MAPK)-family members (p38α, p38β and p38δ) are expressed by immune and inflammatory cells, and are activated by extracellular stimuli such as stress (for example, hypoxia, reactive oxygen species and changes in osmolarity) and pro-inflammatory cytokines (for example, interleukin-1, tumour-necrosis factor and transforming growth factor-β). Although similar in many ways, p38-family members have different tissue distributions and fine specificity for substrates.
-
Studies using specific chemical inhibitors and gene targeting of activators upstream of p38α and p38β have revealed important roles for these kinases in both the production of, and response to, pro-inflammatory stimuli. Little is known about the biological role of p38δ.
-
In the MAPK cascade, p38-family members are activated by serial phosphorylation of upstream kinases that culminate in p38 dual phosphorylation of the Thr-Gly-Tyr motif in the activation loop.
-
p38α specifically binds to and is activated by TAK1-binding protein 1 (TAB1). A hallmark of this activation pathway is p38α autophosphorylation of its own Thr-Gly-Tyr motif.
-
In T cells, T-cell-receptor-induced activation of ζ-chain-associated protein kinase of 70 kDa (ZAP70) leads to p38α/p38β phosphorylation of Tyr323, which induces robust autophosphorylation of the Thr-Gly-Tyr motif and increased activity towards third-party substrates.
-
The activity of spontaneously Tyr323-phosphorylated p38α/p38β is normally regulated by growth arrest and DNA-damage-inducible 45α (GADD45α), and in its absence mice develop T-cell hyperproliferation and autoimmunity.
Abstract
Signals emanating from many cell-surface receptors and environmental cues converge on mitogen-activated protein kinases (MAPKs), which in turn phosphorylate and activate various transcription factors and other molecular effectors. Members of the p38 MAPK family, which respond to pro-inflammatory cytokines and cellular stresses, are typically activated by serial phosphorylation and activation of upstream kinases (the MAPK cascade). In this Review, I highlight the recent studies that indicate that p38-subfamily members can also be activated by non-canonical mechanisms, at least one of which seems to have an important role in antigen-receptor-activated T cells. These alternative pathways might have particular relevance for cells that participate in immune and inflammatory responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Crews, C. M., Alessandrini, A. A. & Erikson, R. L. Mouse Erk-1 gene product is a serine/threonine protein kinase that has the potential to phosphorylate tyrosine. Proc. Natl Acad. Sci. USA 88, 8845–8849 (1991).
Ono, K. & Han, J. The p38 signal transduction pathway: activation and function. Cell. Signal. 12, 1–13 (2000).
Freshney, N. W. et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78, 1039–1049 (1994).
Rouse, J. et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78, 1027–1037 (1994).
Raingeaud, J. et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270, 7420–7426 (1995).
Hannigan, M., Zhan, L., Ai, Y. & Huang, C. K. The role of p38 MAP kinase in TGF-β1-induced signal transduction in human neutrophils. Biochem. Biophys. Res. Commun. 246, 55–58 (1998).
Wilson, K. P. et al. Crystal structure of p38 mitogen-activated protein kinase. J. Biol. Chem. 271, 27696–27700 (1996).
Canagarajah, B. J., Khokhlatchev, A., Cobb, M. H. & Goldsmith, E. J. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90, 859–869 (1997).
Bellon, S., Fitzgibbon, M. J., Fox, T., Hsiao, H. M. & Wilson, K. P. The structure of phosphorylated p38γ is monomeric and reveals a conserved activation-loop conformation. Structure 7, 1057–1065 (1999).
Songyang, Z. et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol. Cell. Biol. 16, 6486–6493 (1996).
Han, J., Lee, J. D., Bibbs, L. & Ulevitch, R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 (1994).
Jiang, Y. et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271, 17920–17926 (1996).
Li, Z., Jiang, Y., Ulevitch, R. J. & Han, J. The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 228, 334–340 (1996).
Kumar, S. et al. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235, 533–538 (1997).
Hale, K. K., Trollinger, D., Rihanek, M. & Manthey, C. L. Differential expression and activation of p38 mitogen-activated protein kinase α, β, γ, and δ in inflammatory cell lineages. J. Immunol. 162, 4246–4252 (1999).
Wu, J. J. & Bennett, A. M. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J. Biol. Chem. 280, 16461–16466 (2005).
Takekawa, M. et al. p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19, 6517–6526 (2000).
Cheung, P. C., Campbell, D. G., Nebreda, A. R. & Cohen, P. Feedback control of the protein kinase TAK1 by SAPK2a/p38α. EMBO J. 22, 5793–5805 (2003).
Lee, J. C., Kassis, S., Kumar, S., Badger, A. & Adams, J. L. p38 mitogen-activated protein kinase inhibitors-mechanisms and therapeutic potentials. Pharmacol. Ther. 82, 389–397 (1999).
Rincon, M. et al. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 17, 2817–2829 (1998).
Haeryfar, S. M. & Hoskin, D. W. Selective pharmacological inhibitors reveal differences between Thy-1- and T cell receptor-mediated signal transduction in mouse T lymphocytes. Int. Immunopharmacol. 1, 689–698 (2001).
Zhang, J. et al. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J. Immunol. 162, 3819–3829 (1999).
Dean, J. L., Sully, G., Clark, A. R. & Saklatvala, J. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell. Signal. 16, 1113–1121 (2004).
Briata, P. et al. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol. Cell 20, 891–903 (2005).
Hitti, E. et al. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol. Cell. Biol. 26, 2399–2407 (2006).
Parasrampuria, D. A., de Boer, P., Desai-Krieger, D., Chow, A. T. & Jones, C. R. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J. Clin. Pharmacol. 43, 406–413 (2003).
Fijen, J. W. et al. Inhibition of p38 mitogen-activated protein kinase: dose-dependent suppression of leukocyte and endothelial response after endotoxin challenge in humans. Crit. Care Med. 30, 841–845 (2002).
Branger, J. et al. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J. Immunol. 168, 4070–4077 (2002).
van den Blink, B. et al. P38 mitogen activated protein kinase is involved in the downregulation of granulocyte CXC chemokine receptors 1 and 2 during human endotoxemia. J. Clin. Immunol. 24, 37–41 (2004).
Kumar, S., Boehm, J. & Lee, J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Rev. Drug Discov. 2, 717–726 (2003).
Palladino, M. A., Bahjat, F. R., Theodorakis, E. A. & Moldawer, L. L. Anti-TNF-α therapies: the next generation. Nature Rev. Drug Discov. 2, 736–746 (2003).
Miwatashi, S. et al. Novel inhibitor of p38 MAP kinase as an anti-TNF-α drug: discovery of N-[4-[2-ethyl-4-(3-methylphenyl)-1, 3-thiazol-5-yl]-2-pyridyl]benzamide (TAK-715) as a potent and orally active anti-rheumatoid arthritis agent. J. Med. Chem. 48, 5966–5979 (2005).
Brancho, D. et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17, 1969–1978 (2003). A genetic analysis of the roles of distinct MAPKKs in the activation of p38α by different stimuli.
Wysk, M., Yang, D. D., Lu, H. T., Flavell, R. A. & Davis, R. J. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl Acad. Sci. USA 96, 3763–3768 (1999).
Tanaka, N. et al. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 3, 785–791 (2002).
Lu, H. T. et al. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18, 1845–1857 (1999).
Bulavin, D. V. et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18, 6845–6854 (1999).
Bulavin, D. V. & Fornace, A. J. J. p38 MAP kinase's emerging role as a tumor suppressor. Adv. Cancer Res. 92, 95–118 (2004).
Brewer, J. A., Kanagawa, O., Sleckman, B. P. & Muglia, L. J. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 169, 1837–1843 (2002).
Suzuki, H. et al. Involvement of MKK6 in TCRαβintCD69lo: a target population for apoptotic cell death in thymocytes. FASEB J. 17, 1538–1540 (2003).
Rincon, M. MAP-kinase signaling pathways in T cells. Curr. Opin. Immunol. 13, 339–345 (2001).
Pedraza-Alva, G. et al. Activation of p38 MAP kinase by DNA double-strand breaks in V(D)J recombination induces a G2/M cell cycle checkpoint. EMBO J. 25, 763–773 (2006).
Lee, J. C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Kuida, K. & Boucher, D. M. Functions of MAP kinases: insights from gene-targeting studies. J. Biochem. (Tokyo) 135, 653–656 (2004).
Adams, R. H. et al. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell. 6, 109–116 (2000).
Kim, J. M., White, J. M., Shaw, A. S. & Sleckman, B. P. MAPK p38α is dispensable for lymphocyte development and proliferation. J. Immunol. 174, 1239–1244 (2005).
Beardmore, V. A. et al. Generation and characterization of p38β (MAPK11) gene-targeted mice. Mol. Cell. Biol. 25, 10454–10464 (2005).
Sabio, G. et al. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145 (2005).
Frantz, B. et al. The activation state of p38 mitogen-activated protein kinase determines the efficiency of ATP competition for pyridinylimidazole inhibitor binding. Biochemistry 37, 13846–13853 (1998).
Galan, A. et al. Stimulation of p38 mitogen-activated protein kinase is an early regulatory event for the cadmium-induced apoptosis in human promonocytic cells. J. Biol. Chem. 275, 11418–11424 (2000).
Zhuang, S., Demirs, J. T. & Kochevar, I. E. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 275, 25939–25948 (2000).
Bowie, A. G. & O'Neill, L. A. Vitamin C inhibits NF-κB activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 165, 7180–7188 (2000).
Matsuguchi, T., Musikacharoen, T., Ogawa, T. & Yoshikai, Y. Gene expressions of Toll-like receptor 2, but not Toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J. Immunol. 165, 5767–5772 (2000).
Park, S. J. & Kim, I. S. The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br. J. Pharmacol. 146, 506–513 (2005).
Barancik, M., Htun, P., Strohm, C., Kilian, S. & Schaper, W. Inhibition of the cardiac p38-MAPK pathway by SB203580 delays ischemic cell death. J. Cardiovasc. Pharmacol. 35, 474–483 (2000).
Park, M. T. et al. Suppression of extracellular signal-related kinase and activation of p38 MAPK are two critical events leading to caspase-8- and mitochondria-mediated cell death in phytosphingosine-treated human cancer cells. J. Biol. Chem. 278, 50624–50634 (2003).
Ge, B. et al. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295, 1291–1294 (2002). This study identified TAB1 as a binding partner and activator of p38α that is independent of the classical MAPK cascade.
Jiang, Y. et al. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J. Biol. Chem. 272, 11096–11102 (1997).
Shibuya, H. et al. TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science 272, 1179–1182 (1996).
Shim, J. H. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 (2005).
Ge, B. et al. TAB1β (transforming growth factor-β-activated protein kinase 1-binding protein 1β), a novel splicing variant of TAB1 that interacts with p38α but not TAK1. J. Biol. Chem. 278, 2286–2293 (2003).
Tanno, M. et al. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ. Res. 93, 254–261 (2003).
Matsuyama, W., Faure, M. & Yoshimura, T. Activation of discoidin domain receptor 1 facilitates the maturation of human monocyte-derived dendritic cells through the TNF receptor associated factor 6/TGF-β-activated protein kinase 1 binding protein 1β/p38α mitogen-activated protein kinase signaling cascade. J. Immunol. 171, 3520–3532 (2003).
Kim, L. et al. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 174, 4178–4184 (2005).
Ohkusu-Tsukada, K., Tominaga, N., Udono, H. & Yui, K. Regulation of the maintenance of peripheral T-cell anergy by TAB1-mediated p38α activation. Mol. Cell. Biol. 24, 6957–6966 (2004).
Ohkusu-Tsukada, K., Tominaga, N., Udono, H. & Yui, K. Erratum: Regulation of the maintenance of peripheral T-cell anergy by TAB1 mediated p38α activation. Mol. Cell. Biol. 25, 8763 (2005).
Salojin, K. V., Zhang, J. & Delovitch, T. L. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J. Immunol. 163, 844–853 (1999).
Yu, H., Leitenberg, D., Li, B. & Flavell, R. A. Deficiency of small GTPase Rac2 affects T cell activation. J. Exp. Med. 194, 915–926 (2001).
Fornace, A. J. Jr et al. Mammalian genes coordinately regulate by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 9, 4196–4203 (1989).
Takekawa, M. & Saito, H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998).
Zhan, Q. et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18, 2892–2900 (1999).
Mita, H., Tsutsui, J., Takekawa, M., Witten, E. A. & Saito, H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 22, 4544–4555 (2002).
Lu, B. et al. GADD45α mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity 14, 583–590 (2001). This study used gene-targeted mice to show a link between a GADD45-family member and p38α and JNK activation in T cells and T H 1-cell differentiation.
Lu, B., Ferrandino, A. F. & Flavell, R. A. Gadd45β is important for perpetuating cognate and inflammatory signals in T cells. Nature Immunol. 5, 38–44 (2004).
Chi, H., Lu, B., Takekawa, M., Davis, R. J. & Flavell, R. A. GADD45β/GADD45γ and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNγ production in T cells. EMBO J. 23, 1576–1586 (2004).
Hollander, M. C. et al. Genomic instability in Gadd45a-deficient mice. Nature Genet. 23, 176–184 (1999).
Bulavin, D. V., Kovalsky, O., Hollander, M. C. & Fornace, A. J. J. Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45 a. Mol. Cell. Biol. 23, 3859–3871 (2003).
Hildesheim, J. et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62, 7305–7315 (2002).
Salvador, J. M. et al. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity 16, 499–508 (2002).
Salvador, J. M., Mittelstadt, P. R., Belova, G. I., Fornace, A. J. Jr & Ashwell, J. D. The autoimmune suppressor Gadd45α inhibits the T cell alternative p38 activation pathway. Nature Immunol. 6, 396–402 (2005). The description of GADD45α as a physiological inhibitor of p38 activated by Tyr323 phosphorylation.
Salvador, J. M. et al. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nature Immunol. 6, 390–395 (2005). This study describes the activation of p38α and p38β by phosphorylation of Tyr323 in the absence of upstream MAPKK activation.
Finco, T. S., Kadlecek, T., Zhang, W., Samelson, L. E. & Weiss, A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 9, 617–626 (1998).
Khokhlatchev, A. V. et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93, 605–615 (1998).
Mittelstadt, P. R., Salvador, J. M., Fornace, A. J. J. & Ashwell, J. D. Activating p38 MAPK: new tricks for an old kinase. Cell Cycle 4, 1189–1192 (2005).
Biondi, R. M. & Nebreda, A. R. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem. J. 372, 1–13 (2003).
Holland, P. M. & Cooper, J. A. Protein modification: docking sites for kinases. Curr. Biol. 9, R329–R331 (1999).
Fantz, D. A., Jacobs, D., Glossip, D. & Kornfeld, K. Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J. Biol. Chem. 276, 27256–27265 (2001).
Gavin, A. C. & Nebreda, A. R. A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr. Biol. 9, 281–284 (1999).
Chang, C. I., Xu, B. E., Akella, R., Cobb, M. H. & Goldsmith, E. J. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell 9, 1241–1249 (2002).
Acknowledgements
I am grateful to P. R. Mittelstadt and R. Bosselut for helpful comments on this manuscript. The Laboratory of Immune Cell Biology is supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute, USA.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Serine/threonine kinases
-
Enzymes that catalyse the phosphorylation of serine or threonine residues in proteins.
- Dual-specificity kinases
-
Enzymes that catalyse the phosphorylation of tyrosine, and serine or threonine residues in proteins.
- Collagen-induced arthritis
-
(CIA). A model of rheumatoid arthritis. CIA develops in susceptible rodents and primates after immunization with cartilage-derived type II collagen.
- Endotoxin-induced septic shock
-
Results from uncontrolled production of pro-inflammatory cytokines owing to bacterial overload. Death might result from multi-organ failure.
- Tetraploid rescue
-
A complementation technique for producing a normal placenta that supports the development of mutant embryos. Briefly, two-cell embryos are fused to create tetraploid cells, which can contribute to all extraembryonic tissues but not to the embryo itself. The tetraploid cells are aggregated with diploid embryonic stem cells or early embryos and implanted into pseudopregnant recipients.
- RAG-deficient blastocyst complementation method
-
A gene-targeting technique used for the genetic analysis of lymphocytes. Complementation of recombination-activating gene 2 (RAG2)-deficient blastocysts (which cannot produce mature T or B cells) with embryonic stem cells that have a mutation in the gene of interest creates chimaeras that have genetically altered T and B cells.
- RNA interference
-
The use of double-stranded RNAs with sequences that precisely match a given gene, to knock-down the expression of that gene by directing RNA-degrading enzymes to destroy the encoded mRNA transcript.
- Vβ8.1-transgenic mice
-
Mice expressing a T-cell receptor (TCR) β-chain of which the variable region is encoded by the 8.1 gene segment. Vβ8.1 binds several superantigens, which results in TCR signalling.
- Superantigen
-
A microbial protein that binds to and activates all T cells that express a particular set of TCR Vβ-chains.
- Aneuploidy
-
The occurrence of one or more extra or missing chromosomes, which leads to an unbalanced chromosome complement.
- Gene amplification
-
The production of multiple copies of a gene or genes.
- Lupus-like autoimmunity
-
An autoimmune disease similar to systemic lupus erythematosus in humans.
- SRC-family kinases
-
A class of cytoplasmic protein tyrosine kinases involved in the initiation of receptor signalling. They include LCK, FYN, LYN, HCK, BLK, C-YES, C-SRC, C-FGR and YRK.
- High-pressure liquid chromatography
-
A rapid variant of column chromatography used for high-resolution separation of molecules of low-to-moderate molecular weight.
Rights and permissions
About this article
Cite this article
Ashwell, J. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol 6, 532–540 (2006). https://doi.org/10.1038/nri1865
Issue Date:
DOI: https://doi.org/10.1038/nri1865
This article is cited by
-
p38 MAPK signaling in chronic obstructive pulmonary disease pathogenesis and inhibitor therapeutics
Cell Communication and Signaling (2023)
-
Bacterial extracellular vesicles repress the vascular protective factor RNase1 in human lung endothelial cells
Cell Communication and Signaling (2023)
-
CNS-Active p38α MAPK Inhibitors for the Management of Neuroinflammatory Diseases: Medicinal Chemical Properties and Therapeutic Capabilities
Molecular Neurobiology (2023)
-
The pro-tumorigenic activity of p38γ overexpression in nasopharyngeal carcinoma
Cell Death & Disease (2022)
-
p38 MAPK Is a Major Regulator of Amyloid Beta-Induced IL-6 Expression in Human Microglia
Molecular Neurobiology (2022)