Key Points
-
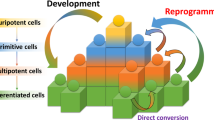
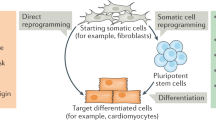
Human induced pluripotent stem (iPS) cells resemble human embryonic stem (ES) cells and have the potential to differentiate into all somatic cell types of the body. Cardiomyocytes and neurons can be derived by using various protocols, but their phenotype is immature, and different subtypes are difficult to obtain and purify.
-
Human iPS cells provide opportunities to capture the heterogeneity that arises from gender, ethnicity and gene modifiers specific to patients from which they have been derived. Although human ES cells have higher genetic stability than human iPS cells, both cell types can be genetically manipulated to generate genotyped matched cell lines, allowing direct comparison of disease-specific genotypes.
-
Both integrating and non-integrating methods are available for reprogramming. Non-integrating approaches are generally preferred but may be less efficient or more labour intensive.
-
iPS cell technology is particularly suitable for modelling diseases and is of special interest for cardiology and neurosciences in which the isolation of primary human tissue is invasive and potentially harmful. Several proof-of-concept studies have recapitulated both genetic and complex diseases of the heart, for example long QT syndrome and catecholaminergic ventricular tachycardia, and the brain, for example, Parkinson's disease, Rett syndrome and schizophrenia.
-
Patient-specific human iPS cell lines are useful for studying drug–gene interactions and for the evaluation of the potency of candidate drugs in reversing aberrant phenotypes that are associated with disease and ameliorating pathological traits. Examples include β-blockade treatment and ion channel activators and inhibitors for cardiac arrhythmogenic disorders, and anti-psychotic drugs or insulin-growth factor application for schizophrenia and autism spectrum disorders, respectively.
-
Availability of larger cohorts of patients, possibly representing different ethnic backgrounds, will help in generalizing effectiveness of therapies and drug adverse effects.
Abstract
Worldwide increases in life expectancy have been paralleled by a greater prevalence of chronic and age-associated disorders, particularly of the cardiovascular, neural and metabolic systems. This has not been met by commensurate development of new drugs and therapies, which is in part owing to the difficulty in modelling human diseases in laboratory assays or experimental animals. Patient-specific induced pluripotent stem (iPS) cells are an emerging paradigm that may address this. Reprogrammed somatic cells from patients are already applied in disease modelling, drug testing and drug discovery, thus enabling researchers to undertake studies for treating diseases 'in a dish', which was previously inconceivable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Rev. Drug Discov. 11, 191–200 (2012).
Mullard, A. 2011 FDA drug approvals. Nature Rev. Drug Discov. 11, 91–94 (2012).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). This breakthrough study demonstrates that pluripotent stem cells, termed iPS cells, can be generated from embryonic and adult tissue cells in mice by expressing only a few specific transcription factors.
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). This study demonstrates the applicability of induced pluripotency technology to human somatic cells.
Burridge, P. W., Keller, G., Gold, J. D. & Wu, J. C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28 (2012).
Liu, H. & Zhang, S. C. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell. Mol. Life Sci. 68, 3995–4008 (2011).
Wu, S. M. & Hochedlinger, K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biol. 13, 497–505 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Stadtfeld, M. & Hochedlinger, K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 24, 2239–2263 (2010).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Gonzalez, F., Boue, S. & Izpisua Belmonte, J. C. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nature Rev. Genet. 12, 231–242 (2011).
Bock, C. et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Ohi, Y. et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature Cell Biol. 13, 541–549 (2011).
Osafune, K. et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nature Biotech. 26, 313–315 (2008).
Murry, C. E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Cheung, C., Bernardo, A. S., Trotter, M. W., Pedersen, R. A. & Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nature Biotech. 30, 165–173 (2012).
Mummery, C. et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107, 2733–2740 (2003).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotech. 25, 1015–1024 (2007).
Xu, X. Q. et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 76, 958–970 (2008).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Mummery, C. L. et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 111, 344–358 (2012).
Dubois, N. C. et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature Biotech. 29, 1011–1018 (2011).
Elliott, D. A. et al. NKX2-5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nature Methods 8, 1037–1040 (2011).
Ritner, C. et al. An engineered cardiac reporter cell line identifies human embryonic stem cell-derived myocardial precursors. PLoS ONE 6, e16004 (2011).
Huber, I. et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 21, 2551–2563 (2007).
Goulburn, A. L. et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells 29, 462–473 (2011).
Noisa, P., Urrutikoetxea-Uriguen, A., Li, M. & Cui, W. Generation of human embryonic stem cell reporter lines expressing GFP specifically in neural progenitors. Stem Cell Rev. 6, 438–449 (2010).
Ma, J. et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J. Physiol. Heart Circ. Physiol. 301, H2006–H2017 (2011).
Zhu, W. Z. et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ. Res. 107, 776–786 (2010).
Hu, B. Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl Acad. Sci. USA 107, 4335–4340 (2010).
Dambrot, C. Passier, R., Atsma, D. & Mummery, C. L. Cardiomyocyte differentiation of pluripotent stem cells and their use as cardiac disease models. Biochem. J. 434, 25–35 (2011).
Lombardi, R. et al. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ. Res. 109, 1342–1353 (2011).
Siedner, S. et al. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J. Physiol. 548, 493–505 (2003).
Schaaf, S. et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS ONE 6, e26397 (2011).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nature Nanotechnol. 6, 720–725 (2011).
Davis, R. P., van den Berg, C. W., Casini, S., Braam, S. R. & Mummery, C. L. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol. Med. 17, 475–484 (2011).
Carvajal-Vergara, X. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010).
Sun, N. et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 4, 130ra147 (2012).
Shi, Y., Kirwan, P., Smith, J., Robinson, H. P. & Livesey, F. J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nature Neurosci. 15, 477–486 (2012).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Brennand, K. J. & Gage, F. H. Concise review: the promise of human induced pluripotent stem cell-based studies of schizophrenia. Stem Cells 29, 1915–1922 (2011).
Muotri, A. R., Nakashima, K., Toni, N., Sandler, V. M. & Gage, F. H. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc. Natl Acad. Sci. USA 102, 18644–18648 (2005).
Jensen, M. B., Yan, H., Krishnaney-Davison, R., Al Sawaf, A. & Zhang, S. C. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J. Stroke Cerebrovasc. Dis. 10 Nov 2011 (doi:10.1016/j.jstrokecerebrovasdis.2011.09.008).
Laurent, L. C. et al. Restricted ethnic diversity in human embryonic stem cell lines. Nature Methods 7, 6–7 (2010).
Narva, E. et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nature Biotech. 28, 371–377 (2010).
Pera, M. F. Stem cells: The dark side of induced pluripotency. Nature 471, 46–47 (2011).
Mummery, C. Induced pluripotent stem cells — a cautionary note. N. Engl. J. Med. 364, 2160–2162 (2011).
Young, M. A. et al. Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell 10, 570–582 (2012).
Yusa, K. et al. Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature 478, 391–394 (2011).
Liu, G. H. et al. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell Stem Cell 8, 688–694 (2011).
Soldner, F. et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977 (2009).
Costa, M. et al. A method for genetic modification of human embryonic stem cells using electroporation. Nature Protoc. 2, 792–796 (2007).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Miller, J. C. et al. A TALE nuclease architecture for efficient genome editing. Nature Biotech. 29, 143–148 (2011).
Soldner, F. et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318–331 (2011).
The Internation HapMap Consortium. The International HapMap Project. Nature 426, 789–796 (2003).
Braam, S. R. et al. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res. 6 Sept 2012 (doi:10.1016/j.scr.2012.08.007).
Tiscornia, G., Vivas, E. L. & Belmonte, J. C. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nature Med. 17, 1570–1576 (2011). Describes the generation of the first iPS cells from patients with various genetic diseases, characterised by either Mendelian or complex inheritance.
Park, I. H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008).
Simons, B. D. & Clevers, H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145, 851–862 (2011).
Matsa, E. et al. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur. Heart J. 32, 952–962 (2011).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010). Generates iPS cells from the skin of two patients with LQTS and shows that their differentiated cardiac derivatives recapitulated the disease phenotype in vitro and that the efficacy of β-blockade therapy was evident in these cells.
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Lahti, A. L. et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis. Model. Mech. 5, 220–230 (2012).
Jung, C. B. et al. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol. Med. 4, 180–191 (2012).
Fatima, A. et al. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell. Physiol. Biochem. 28, 579–592 (2011).
Novak, A. et al. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J. Cell. Mol. Med. 16, 468–482 (2012).
Davis, R. P. et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 125, 3079–3091 (2012).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009). Uses iPS cells from patients that are affected by familial dysautonomia to extend the knowledge and to provide new insights into the disease: mis-splicing of mutated IKBKAP occurred in a tissue-specific way; neural crest precursors expressed low levels of ASCL1 (achaete-scute complex homologue 1), resulting in a primary defect of autonomic neurogenesis; neural crest precursors had migration defects; and kinetin could partially rescue the splicing defect in human iPS cell-derived peripheral neurons.
Marchetto, M. C. et al. A model for neural development and treatment of RTT using human induced pluripotent stem cells. Cell 143, 527–539 (2010). Derives iPS cells from patients that are affected by RTT and differentiates these cells into neurons that showed abnormalities in vitro that could be rescued by specific drug treatment.
Batista, L. F. et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399–402 (2011).
Devine, M. J. et al. Parkinson's disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nature Commun. 2, 440 (2011).
Brennand, K. J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011). Generates iPS cells from patients that are affected by schizophrenia and differentiated these cells into neurons showing phenotypic and gene expression changes that were ameliorated upon anti-psychotic drug application.
Pasca, S. P. et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nature Med. 17, 1657–1662 (2011).
Koch, P. et al. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado–Joseph disease. Nature 480, 543–546 (2011).
Israel, M. A. et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature 482, 216–220 (2012).
Faustino, N. A. & Cooper, T. A. Pre-mRNA splicing and human disease. Genes Dev. 17, 419–437 (2003).
Mekhoubad, S. et al. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 10, 595–609 (2012).
Vogel, G. Stem cells. Diseases in a dish take off. Science 330, 1172–1173 (2010).
Gore, A. et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471, 63–67 (2011).
Marchetto, M. C. et al. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS ONE 4, e7076 (2009).
Bruck, T. & Benvenisty, N. Meta-analysis of the heterogeneity of X chromosome inactivation in human pluripotent stem cells. Stem Cell Res. 6, 187–193 (2011).
Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. & Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 85, 348–362 (2009).
The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Kaji, K. et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458, 771–775 (2009).
Woltjen, K., Hamalainen, R., Kibschull, M., Mileikovsky, M. & Nagy, A. Transgene-free production of pluripotent stem cells using piggyBac transposons. Methods Mol. Biol. 767, 87–103 (2011).
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G. & Hochedlinger, K. Induced pluripotent stem cells generated without viral integration. Science 322, 945–949 (2008).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Miyoshi, N. et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 8, 633–638 (2011).
Kim, D. et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4, 472–476 (2009).
Zhu, S. et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7, 651–655 (2010).
Esteban, M. A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Lemonnier, T. et al. Modeling neuronal defects associated with a lysosomal disorder using patient-derived induced pluripotent stem cells. Hum. Mol. Genet. 20, 3653–3666 (2011).
Kazuki, Y. et al. Complete genetic correction of iPS cells from Duchenne muscular dystrophy. Mol. Ther. 18, 386–393 (2010).
Urbach, A., Bar-Nur, O., Daley, G. Q. & Benvenisty, N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell 6, 407–411 (2010).
Jang, J. et al. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann. Neurol. 70, 402–409 (2011).
Ho, J. C. et al. Generation of induced pluripotent stem cell lines from 3 distinct laminopathies bearing heterogeneous mutations in lamin A/C. Aging (Albany NY) 3, 380–390 (2011).
Ye, Z. et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114, 5473–5480 (2009).
Maehr, R. et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl Acad. Sci. USA 106, 15768–15773 (2009).
Ghodsizadeh, A. et al. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev. 6, 622–632 (2010).
Agarwal, S. et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 464, 292–296 (2010).
Tolar, J. et al. Induced pluripotent stem cells from individuals with recessive dystrophic epidermolysis bullosa. J. Invest. Dermatol. 131, 848–856 (2011).
Itoh, M., Kiuru, M., Cairo, M. S. & Christiano, A. M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 108, 8797–8802 (2011).
Mitne-Neto, M. et al. Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum. Mol. Genet. 20, 3642–3652 (2011).
Dimos, J. T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008).
Rashid, S. T. et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J. Clin. Invest. 120, 3127–3136 (2010).
Zhang, J. et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31–45 (2011).
Liu, G. H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472, 221–225 (2011).
Tolar, J. et al. Hematopoietic differentiation of induced pluripotent stem cells from patients with mucopolysaccharidosis type I (Hurler syndrome). Blood 117, 839–847 (2011).
Nguyen, H. N. et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280 (2011).
Seibler, P. et al. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J. Neurosci. 31, 5970–5976 (2011).
Swistowski, A. et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28, 1893–1904 (2010).
Jin, Z. B. et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS ONE 6, e17084 (2011).
Muotri, A. R. et al. L1 retrotransposition in neurons is modulated by MeCP2. Nature 468, 443–446 (2010).
Zhang, S. et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum. Mol. Genet. 20, 3176–3187 (2011).
Zou, J. et al. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood 117, 5561–5572 (2011).
Pedrosa, E. et al. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J. Neurogenet. 25, 88–103 (2011).
Paulsen, B. D. et al. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. 22 Sept 2011 (doi:10.3727/096368911X600957).
Camnasio, S. et al. The first reported generation of several induced pluripotent stem cell lines from homozygous and heterozygous Huntington's disease patients demonstrates mutation related enhanced lysosomal activity. Neurobiol. Dis. 46, 41–51 (2012).
Jeon, I. et al. Neuronal properties, in vivo effects and pathology of a Huntington's Disease patient-derived induced pluripotent stem cells. Stem Cells 30, 2054–2062 (2012).
Liu, J. et al. Signaling defects in iPSC-derived fragile X premutation neurons. Hum. Mol. Genet. 21, 3795–3805 (2012).
Egawa, N. et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 4, 145ra104 (2012).
Ma, D. et al. Generation of patient-specific induced pluripotent stem cell-derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 13 Jul 2012 (doi:10.1093/eurheartj/ehs226).
Egashira, T. et al. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc. Res. 95, 419–429 (2012).
Andrade, L. N., Nathanson, J. L., Yeo, G. W., Menck, C. F. & Muotri, A. R. Evidence for premature aging due to oxidative stress in iPSCs from Cockayne Syndrome. Hum. Mol. Genet. 21, 3825–3834 (2012).
Hotta, A., et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nature Methods 6, 370–376 (2009).
Bilican, B., et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc. Natl Acad. Sci. USA 109, 5803–5808 (2012).
Yagi, T., et al. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum. Mol. Genet. 20, 4530–4539 (2011).
Ananiev, G., Williams, E. C., Li, H. & Chang, Q. Isogenic pairs of wild type and mutant induced pluripotent stem cell (iPSC) lines from Rett syndrome patients as in vitro disease model. PloS one 6, e25255 (2011).
Cheung, A. Y., et al. Isolation of MECP2-null Rett syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum. Mol. Genet. 20, 2103–2115 (2011).
Huang, H. P., et al. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum. Mol. Genet. 20, 4851–4864 (2011).
Ricciardi, S., et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nature Cell Biol. 14, 911–923 (2012).
Somers, A., et al. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 28, 1728–1740 (2010).
Khan, I. F., et al. Engineering of human pluripotent stem cells by AAV-mediated gene targeting. Mol. Ther. 18, 1192–1199 (2010).
Hargus, G., et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl Acad. Sci. USA 107, 15921–15926 (2010).
Ebert, A. D., et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009).
Howden, S. E., et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc. Natl Acad. Sci. USA 108, 6537–6542 (2011).
Chiang, C. H., et al. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol. Psychiatry 16, 358–360 (2011).
Boulting, G. L., et al. A functionally characterized test set of human induced pluripotent stem cells. Nature Biotechnol. 29, 279–286 (2011).
Braam, S. R., et al. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 4, 107–116 (2010).
Son, E. Y., et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 (2011).
Marchetto, M. C., et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649–657 (2008).
Gold-von Simson, G., et al. Kinetin in familial dysautonomia carriers: implications for a new therapeutic strategy targeting mRNA splicing. Pediatr. Res. 65, 341–346 (2009).
Axelrod, F. B., et al. Kinetin improves IKBKAP mRNA splicing in patients with familial dysautonomia. Pediatr. Res. 70, 480–483 (2011).
Pini, G., et al. IGF1 as a potential treatment for Rett syndrome: safety assessment in six Rett patients. Autism research and treatment 2012, Article ID 679801 (2012).
Zhang, Q., et al. Preparation of uniaxial multichannel silk fibroin scaffolds for guiding primary neurons. Acta Biomater. 8, 2628–2638 (2012).
Major, T., et al. Transgene excision has no impact on in vivo integration of human iPS derived neural precursors. PloS one 6, e24687 (2011).
Zhang, N., An, M. C., Montoro, D. & Ellerby, L. M. Characterization of human Huntington's disease cell model from induced pluripotent stem cells. PLoS currents 2, RRN1193 (2010).
Acknowledgements
The authors thank L. Studer for sharing insights on the familial dysautonomia human induced pluripotent stem cell model and R.P. Davis for constructive comments on the manuscript. Work in the Mummery laboratory was supported by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908) and M.B. is supported by the EU Marie Curie FP7-people-2011-IEF programme (HPSCLQT No. 29999).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Christine L. Mummery's homepage
Coriell Institute Biobank Catalog
California Institute for Regenerative Medicine (CIRM)
Innovative Medicine Initiative (IMI)
Center for iPS Cell Research and Application (CiRA) Kyoto University
Core Facility Activities, Rutgers The State University of New Jersey
Glossary
- Somatic cell nuclear transfer
-
Reprogramming technique in which a nucleus from a differentiated somatic cell is transplanted into an enucleated oocyte.
- Cell co-culture system
-
In vitro differentiation technique in which pluripotent stem cells are cultured together with another cell type that is able to induce and support the specification of a particular lineage by mimicking in vivo tissue niches.
- Neurite
-
A projection from the cell body of a neuron.
- Homologous recombination
-
A type of genetic recombination in which DNA is exchanged between two DNA molecules that share high sequence similarity. This process is exploited for genetic manipulation of mouse and human embryonic stem cells.
- Glial cells
-
Non-neuronal cells that provide support and protection for neurons in the brain and in other parts of the nervous system.
- Sarcomere
-
The contractile unit of the skeletal and cardiac muscle fibre that consists of several contractile proteins.
- Cortical neurons
-
Nerve cells that make up the cerebral cortex, which is the outer part of the brain.
- Synapses
-
Structures that, in the nervous system, allow a neuron to communicate with another cell. The transmitted signal can be electrical or chemical (neurotransmitter).
- Zinc-finger nucleases
-
(ZNFs). Artificial proteins that contain a sequence-specific DNA-binding domain (composed of engineered zinc-finger arrays) fused to a nuclease domain (from the FokI restriction enzyme). ZNFs cleave DNA in a non-sequence-specific manner.
- Transcription activator-like effector nucleases
-
(TALENs). Like ZFNs, they consist of a non-specific FokI nuclease domain fused to a DNA-binding domain derived from bacterial transcription activator-like effectors (TALEs).
- Adult progenitor cells
-
Self-renewing precursor cells that are present in some adult tissues and that are able to produce one or more specialized cell types.
- Long QT syndromes
-
(LQTSs). Channelopathies characterized by a prolongation of the QT interval in the electrocardiogram and a propensity to ventricular tachycardia and sudden death. These syndromes are not associated with concomitant structural cardiac abnormalities.
- Brugada syndromes
-
Channelopathies characterized by a specific electrocardiographic pattern and susceptibility to ventricular arrhythmia and sudden death. These syndromes are not associated with concomitant structural cardiac abnormalities.
- Action potential duration
-
(APD). Duration of the event in which the membrane potential of an electrically excitable cell rises and falls.
- Inward Na+ current
-
The specific Na+ current that passes through the Na+ channel SCN5A. This current is present at the surface of cardiac cells and is essential for the beginning of an action potential.
- Familial dysautonomia
-
A rare but fatal peripheral neuropathy that is characterized by the degeneration and depletion of autonomic and sensory neurons.
- Rett syndrome
-
(RTT). A progressive neurological disorder in which patients show a large spectrum of autistic characteristics.
- Penetrance
-
In genetics, the proportion of individuals within a population carrying a mutation that causes a particular trait and that exhibits the specific associated clinical symptoms.
- Roscovitine
-
A compound that selectively inhibits cyclin-dependent kinases and increases the (voltage-dependent) inactivation of the l-type Ca2+ channel CACNA1C.
- Calpain
-
A family of Ca2+-dependent proteases that has an important role in the signal transduction pathway by catalysing the controlled proteolysis of target proteins.
- Ataxin 3
-
(ATXN3). A deubiquitylating enzyme that is involved in protein homeostasis maintenance, transcription, cytoskeleton regulation, myogenesis and degradation of misfolded chaperone substrates.
Rights and permissions
About this article
Cite this article
Bellin, M., Marchetto, M., Gage, F. et al. Induced pluripotent stem cells: the new patient?. Nat Rev Mol Cell Biol 13, 713–726 (2012). https://doi.org/10.1038/nrm3448
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3448
This article is cited by
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy
Molecular Cancer (2023)
-
Advances in stem cell research for the treatment of primary hypogonadism
Nature Reviews Urology (2021)
-
Genome-wide CRISPR/Cas9 screening in human iPS derived cardiomyocytes uncovers novel mediators of doxorubicin cardiotoxicity
Scientific Reports (2021)
-
Human forebrain endothelial cell therapy for psychiatric disorders
Molecular Psychiatry (2021)
-
In vitro monitoring of HTR2A-positive neurons derived from human-induced pluripotent stem cells
Scientific Reports (2021)