Key Points
-
The brain depends on a continuous supply of oxygen, so it is crucial for the body to be able to detect and respond rapidly to hypoxia. This review highlights the central role of the transcription factor hypoxia-inducible factor 1 (HIF1) in hypoxia sensing at the cellular level.
-
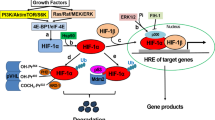
HIF1 consists of two subunits — HIF1α and HIF1β. HIF1β is expressed constitutively in all cells and does not respond to changes in oxygen tension, but it is essential for hypoxia-induced transcriptional changes mediated by the HIF1 heterodimer. HIF1α is made continuously and accumulates in hypoxic cells, but is rapidly degraded and is virtually absent in normoxic cells.
-
HIF1 regulates the expression of a range of genes that facilitate acclimatization to low oxygen conditions. Its targets include genes that code for molecules that participate in vasomotor control, angiogenesis, erythropoiesis, iron metabolism, cell proliferation and cell cycle control, cell death, and energy metabolism.
-
Under hypoxic conditions, prolyl- and asparaginyl-hydroxylases are inhibited, thereby preventing hydroxylation of HIF1α. Inhibiting prolyl-hydroxylases decreases degradation of HIF1α, leading to rapid HIF1α protein accumulation. The HIF1α protein is phosphorylated and dimerizes with HIF1β. The HIF1α/HIF1β dimer binds p300/CBP, thereby activating hypoxia response elements in HIF target genes.
-
HIF1 has been implicated in a range of brain pathologies, and it can be harmful or beneficial, depending on the circumstances. HIF1 has been implicated not only in conditions that can be directly attributed to hypoxia, such as cerebral and retinal ischaemia, but also in diseases with less well-defined aetiologies.
-
Hypoxic preconditioning, or hypoxia-induced tolerance, refers to a brief period of hypoxia that protects against an otherwise lethal subsequent insult, such as stroke. It has been proposed that preconditioning is due, at least in part, to hypoxic induction of HIF1 and HIF1 target genes.
-
The ability to modify the HIF1 pathway using specific tools, such as prolyl-hydroxylase inhibitors, might facilitate the development of pharmaceutical strategies to treat pathologies in which HIF1 activation might be beneficial. By contrast, strategies for blocking the HIF1 pathway might be useful for treating brain tumours in which a poor prognosis has been attributed to the hypoxic status.
Abstract
Of all the chemical elements, oxygen is the most vital to the human body. The brain is the most sensitive organ to oxygen deprivation (hypoxia), which, over an extended period, can cause coma, seizures, cognitive impairment and other neurological disabilities, and even brain death. However, during mild hypoxia of short duration, the brain develops adaptative mechanisms that allow it to maintain normal physiological conditions. In this review, we discuss some of the molecular mechanisms of oxygen sensing in the brain. Particular emphasis is placed on the oxygen-dependant regulation of the transcription factor HIF1 (hypoxia-inducible factor 1) — one of the main cellular responses to hypoxia that operates in numerous cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Silver, I. & Erecinska, M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv. Exp. Med. Biol. 454, 7–16 (1998).
Ray, C. J., Abbas, M. R., Coney, A. M. & Marshall, J. M. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J. Physiol. (Lond.) 544, 195–209 (2002).
Van Mil, A. H. et al. Nitric oxide mediates hypoxia-induced cerebral vasodilation in humans. J. Appl. Physiol. 92, 962–966 (2002).
Peers, C. Oxygen-sensitive ion channels. Trends Pharmacol. Sci. 18, 405–408 (1997).
Wang, G. L., Jiang, B. H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514 (1995).
Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J. Biol. Chem. 271, 15117–15123 (1996).
Jiang, B. H., Semenza, G. L., Bauer, C. & Marti, H. H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271, C1172–1180 (1996).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998).
Ema, M. et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl Acad. Sci. USA 94, 4273–4278 (1997). References 9 and 10 provided the first evidence of the HIF1α-related protein HIF2α.
Tian, H., McKnight, S. L. & Russell, D. W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11, 72–82 (1997).
Gu, Y. Z., Moran, S. M., Hogenesch, J. B., Wartman, L. & Bradfield, C. A. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 7, 205–213 (1998). These authors cloned the cDNA that encodes HIF3α and showed that it binds to HIF1β, is activated by hypoxia and binds as a heterodimer to the HRE sequence.
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162 (1998).
Ryan, H. E., Lo, J. & Johnson, R. S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3015 (1998).
Tian, H., Hammer, R. E., Matsumoto, A. M., Russell, D. W. & McKnight, S. L. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 (1998).
Peng, J., Zhang, L., Drysdale, L. & Fong, G. H. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc. Natl Acad. Sci. USA 97, 8386–8391 (2000).
Compernolle, V. et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nature Med. 8, 702–710 (2002).
Wiesener, M. S. et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 17, 271–273 (2003).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J. Biol. Chem. 277, 32405–32408 (2002).
Ruscher, K. et al. Induction of hypoxia inducible factor 1 by oxygen glucose deprivation is attenuated by hypoxic preconditioning in rat cultured neurons. Neurosci. Lett. 254, 117–120 (1998).
Chavez, J. C., Agani, F., Pichiule, P. & LaManna, J. C. Expression of hypoxia-inducible factor-1α in the brain of rats during chronic hypoxia. J. Appl. Physiol. 89, 1937–1942 (2000).
Ralph, G. S. et al. Identification of potential stroke targets by lentiviral vector mediated overexpression of HIF-1α and HIF-2α in a primary neuronal model of hypoxia. J. Cereb. Blood Flow Metab. 24, 245–258 (2004).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Yu, A. Y. et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Invest. 103, 691–696 (1999). A study that provides, with the use of total or partial HIF1 deficiency in mice, definitive evidence that HIF1 is essential for normal embryonic development. It also provides a definitive connection between HIF1 expression and physiological responses to hypoxia in adult animals.
Tomita, S. et al. Defective brain development in mice lacking the Hif-1α gene in neural cells. Mol. Cell. Biol. 23, 6739–6749 (2003). These results show that expression of HIF1α in neural cells is essential for normal development of the brain.
Kojima, H. et al. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1α-deficient chimeric mice. Proc. Natl Acad. Sci. USA 99, 2170–2174 (2002).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Bruick, R. K. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc. Natl Acad. Sci. USA 97, 9082–9087 (2000).
Sowter, H. M., Ratcliffe, P. J., Watson, P., Greenberg, A. H. & Harris, A. L. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 61, 6669–6673 (2001).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604–4613 (1996).
Gerber, H. P., Condorelli, F., Park, J. & Ferrara, N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 272, 23659–23667 (1997).
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Ebert, B. L., Firth, J. D. & Ratcliffe, P. J. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J. Biol. Chem. 270, 29083–29089 (1995).
Cormier-Regard, S., Nguyen, S. V. & Claycomb, W. C. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J. Biol. Chem. 273, 17787–17792 (1998).
Takahashi, Y., Takahashi, S., Shiga, Y., Yoshimi, T. & Miura, T. Hypoxic induction of prolyl 4-hydroxylase α (I) in cultured cells. J. Biol. Chem. 275, 14139–14146 (2000).
Palmer, L. A., Semenza, G. L., Stoler, M. H. & Johns, R. A. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am. J. Physiol. 274, L212–219 (1998).
Eckhart, A. D., Yang, N., Xin, X. & Faber, J. E. Characterization of the α1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc. Natl Acad. Sci. USA 94, 9487–9492 (1997).
Hu, J., Discher, D. J., Bishopric, N. H. & Webster, K. A. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem. Biophys. Res. Commun. 245, 894–899 (1998).
Kietzmann, T., Roth, U. & Jungermann, K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood 94, 4177–4185 (1999).
Schaffer, L. et al. Oxygen-regulated expression of TGF-β3, a growth factor involved in trophoblast differentiation. Placenta 24, 941–950 (2003).
Rolfs, A., Kvietikova, I., Gassmann, M. & Wenger, R. H. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J. Biol. Chem. 272, 20055–20062 (1997).
Tacchini, L., Bianchi, L., Bernelli-Zazzera, A. & Cairo, G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 274, 24142–24146 (1999).
Mukhopadhyay, C. K., Mazumder, B. & Fox, P. L. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J. Biol. Chem. 275, 21048–21054 (2000).
Bhattacharya, S. et al. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 13, 64–75 (1999).
Feldser, D. et al. Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res. 59, 3915–3918 (1999).
Tazuke, S. I. et al. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc. Natl Acad. Sci. USA 95, 10188–10193 (1998).
Lee, P. J. et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 272, 5375–5381 (1997).
O'Rourke, J. F., Pugh, C. W., Bartlett, S. M. & Ratcliffe, P. J. Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur. J. Biochem. 241, 403–410 (1996).
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994).
Kaluz, S. et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1α stabilization: a role for phosphatidylinositol 3'-kinase. Cancer Res. 62, 4469–4477 (2002).
Graven, K. K., Yu, Q., Pan, D., Roncarati, J. S. & Farber, H. W. Identification of an oxygen responsive enhancer element in the glyceraldehyde-3-phosphate dehydrogenase gene. Biochim. Biophys. Acta 1447, 208–218 (1999).
Shoshani, T. et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol. Cell. Biol. 22, 2283–2293 (2002).
Miyazaki, K. et al. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J. Biol. Chem. 277, 47014–47021 (2002).
Seta, K. A., Kim, R., Kim, H. W., Millhorn, D. E. & Beitner-Johnson, D. Hypoxia-induced regulation of MAPK phosphatase-1 as identified by subtractive suppression hybridization and cDNA microarray analysis. J. Biol. Chem. 276, 44405–44412 (2001).
Burmester, T., Weich, B., Reinhardt, S. & Hankeln, T. A vertebrate globin expressed in the brain. Nature 407, 520–523 (2000). This study reports the identification of a third globin type in human and mouse, predominantly expressed in the brain and therefore called neuroglobin, which might help to transport oxygen across the blood–brain barrier and increase the availability of oxygen to the metabolically active neuronal tissues.
Pichiule, P., Chavez, J. C. & LaManna, J. C. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 279, 12171–12180 (2003).
Pichiule, P. & LaManna, J. C. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J. Appl. Physiol. 93, 1131–1139 (2002).
Metzen, E. et al. Intracellular localisation of human HIF-1α hydroxylases: implications for oxygen sensing. J. Cell Sci. 116, 1319–1326 (2003).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). The authors show that HIF1 is regulated through prolyl-hydroxylation at a conserved core LXXLAP motif, and define the novel class of HIF1 prolyl-hydroxylases (PHDs), providing the basis for an oxygen-sensing function.
Bruick, R. K. & McKnight, S. L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 (2001). The same year as reference 58, these authors also revealed a family of HIF1 prolyl-hydroxylases named HPHs, which seemed to be identical to the PHD class of HIF1 prolyl-hydroxylase described by Epstein et al.
Taylor, M. S. Characterization and comparative analysis of the EGLN gene family. Gene 275, 125–132 (2001).
Oehme, F. et al. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem. Biophys. Res. Commun. 296, 343–349 (2002).
Mole, D. R., Maxwell, P. H., Pugh, C. W. & Ratcliffe, P. J. Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing. IUBMB Life 52, 43–47 (2001).
Hon, W. C. et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature 417, 975–978 (2002).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Masson, N., Willam, C., Maxwell, P. H., Pugh, C. W. & Ratcliffe, P. J. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 (2001).
Min, J. H. et al. Structure of an HIF-1α–pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002).
Kibel, A., Iliopoulos, O., DeCaprio, J. A. & Kaelin, W. G. Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 269, 1444–1446 (1995).
Pause, A. et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl Acad. Sci. USA 94, 2156–2161 (1997).
Iwai, K. et al. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl Acad. Sci. USA 96, 12436–12441 (1999).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). The first evidence that VHL binds to HIF1 and HIF2 and targets them for destruction.
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Huang, L. E. & Bunn, H. F. Hypoxia-inducible factor and its biomedical relevance. J. Biol. Chem. 278, 19575–19578 (2003).
Ratcliffe, P. J., O'Rourke, J. F., Maxwell, P. H. & Pugh, C. W. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J. Exp. Biol. 201, 1153–1162 (1998).
Wang, G. L. & Semenza, G. L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood 82, 3610–3615 (1993).
Yuan, Y., Hilliard, G., Ferguson, T. & Millhorn, D. E. Cobalt inhibits the interaction between hypoxia-inducible factor-α and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-α. J. Biol. Chem. 278, 15911–15916 (2003). Until this study, cobalt had been thought to activate HIF1 through HIF1 prolyl-hydroxylase inhibition. Here, the authors show that cobalt can also inhibit VHL binding to HIFα by direct binding to the VHL-binding domain.
Jeong, J. W. et al. Regulation and destabilization of HIF-1α by ARD1-mediated acetylation. Cell 111, 709–720 (2002).
Isaacs, J. S. et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J. Biol. Chem. 277, 29936–29944 (2002).
Mahon, P. C., Hirota, K. & Semenza, G. L. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 (2001). The identification and characterization of FIH1 (factor inhibiting HIF1), a negative regulator of HIF1 transactivation-domain function. The authors demonstrate that FIH1 interacts with HIF1, as well as with VHL, and that both FIH1 and VHL inhibit HIF1 transactivation-domain function, establishing a unifying mechanism for the modulation of HIF1 protein stabilization and transcriptional activation in response to changes in cellular O 2 concentration.
Hewitson, K. S. et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J. Biol. Chem. 277, 26351–26355 (2002).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Elkins, J. M. et al. Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1α. J. Biol. Chem. 278, 1802–1806 (2003).
Freedman, S. J. et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1α. Proc. Natl Acad. Sci. USA 99, 5367–5372 (2002).
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J. & Whitelaw, M. L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295, 858–861 (2002).
Huang, J., Zhao, Q., Mooney, S. M. & Lee, F. S. Sequence determinants in hypoxia-inducible factor-1α for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J. Biol. Chem. 277, 39792–39800 (2002).
Lipscomb, E. A., Sarmiere, P. D. & Freeman, R. S. SM-20 is a novel mitochondrial protein that causes caspase-dependent cell death in nerve growth factor-dependent neurons. J. Biol. Chem. 276, 5085–5092 (2001).
Lieb, M. E., Menzies, K., Moschella, M. C., Ni, R. & Taubman, M. B. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem. Cell Biol. 80, 421–426 (2002).
Lipscomb, E. A., Sarmiere, P. D., Crowder, R. J. & Freeman, R. S. Expression of the SM-20 gene promotes death in nerve growth factor-dependent sympathetic neurons. J. Neurochem. 73, 429–432 (1999).
Semenza, G. L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7, 345–350 (2001).
Agani, F. H., Pichiule, P., Chavez, J. C. & LaManna, J. C. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J. Biol. Chem. 275, 35863–35867 (2000).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Vaux, E. C., Metzen, E., Yeates, K. M. & Ratcliffe, P. J. Regulation of hypoxia-inducible factor is preserved in the absence of a functioning mitochondrial respiratory chain. Blood 98, 296–302 (2001).
Bergeron, M., Yu, A. Y., Solway, K. E., Semenza, G. L. & Sharp, F. R. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur. J. Neurosci. 11, 4159–4170 (1999). The first study reporting HIF1 upregulation in the brain after focal cerebral ischaemia.
Bergeron, M. et al. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann. Neurol. 48, 285–296 (2000).
Semenza, G. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 64, 993–998 (2002).
Fukuda, R. et al. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 277, 38205–38211 (2002).
Laughner, E., Taghavi, P., Chiles, K., Mahon, P. C. & Semenza, G. L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21, 3995–4004 (2001).
Chavez, J. C. & LaManna, J. C. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J. Neurosci. 22, 8922–8931 (2002).
Jiang, B. H. et al. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 12, 363–369 (2001).
Xi, G., Reiser, G. & Keep, R. F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J. Neurochem. 84, 3–9 (2003).
Lu, H., Forbes, R. A. & Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 277, 23111–23115 (2002).
Dalgard, C., Lu, H., Mohyeldin, A. & Verma, A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem. J. 25 Feb 2004 (doi:10.1042/BJ20031647)
Metzen, E., Zhou, J., Jelkmann, W., Fandrey, J. & Brune, B. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell 14, 3470–3481 (2003).
Hagen, T., Taylor, C. T., Lam, F. & Moncada, S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302, 1975–1978 (2003).
Arsham, A. M., Howell, J. J. & Simon, M. C. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278, 29655–29660 (2003).
Hudson, C. C. et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22, 7004–7014 (2002).
Marti, H. J. et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 156, 965–976 (2000).
Pichiule, P., Agani, F., Chavez, J. C., Xu, K. & LaManna, J. C. HIF-1α and VEGF expression after transient global cerebral ischemia. Adv. Exp. Med. Biol. 530, 611–617 (2003).
Iadecola, C., Zhang, F., Xu, S., Casey, R. & Ross, M. E. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J. Cereb. Blood Flow Metab. 15, 378–384 (1995).
Wang, X. et al. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc. Natl Acad. Sci. USA 92, 11480–11484 (1995).
Serrano, J. et al. Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience 109, 717–731 (2002).
Encinas, J. M., Serrano, J., Alonso, D., Fernandez, A. P. & Rodrigo, J. Adrenomedullin over-expression in the caudate-putamen of the adult rat brain after ischaemia-reperfusion injury. Neurosci. Lett. 329, 197–200 (2002).
Zhang, F., White, J. G. & Iadecola, C. Nitric oxide donors increase blood flow and reduce brain damage in focal ischemia: evidence that nitric oxide is beneficial in the early stages of cerebral ischemia. J. Cereb. Blood Flow Metab. 14, 217–226 (1994).
Dogan, A. et al. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 17, 19–25 (1997).
Watanabe, K. et al. Adrenomedullin reduces ischemic brain injury after transient middle cerebral artery occlusion in rats. Acta Neurochir. (Wien) 143, 1157–1161 (2001).
Dalkara, T., Yoshida, T., Irikura, K. & Moskowitz, M. A. Dual role of nitric oxide in focal cerebral ischemia. Neuropharmacology 33, 1447–1452 (1994).
Kim, W. et al. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J. 17, 1937–1939 (2003).
Hayashi, T., Abe, K., Suzuki, H. & Itoyama, Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke 28, 2039–2044 (1997).
Lennmyr, F., Ata, K. A., Funa, K., Olsson, Y. & Terent, A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J. Neuropathol. Exp. Neurol. 57, 874–882 (1998).
Docagne, F. et al. Transforming growth factor-β1 as a regulator of the serpins/t-PA axis in cerebral ischemia. FASEB J. 13, 1315–1324 (1999).
Krupinski, J., Kaluza, J., Kumar, P., Kumar, S. & Wang, J. M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25, 1794–1798 (1994).
O'Rourke, J. F. et al. Hypoxia response elements. Oncol. Res. 9, 327–332 (1997).
Semenza, G. L., Nejfelt, M. K., Chi, S. M. & Antonarakis, S. E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc. Natl Acad. Sci. USA 88, 5680–5684 (1991).
Semenza, G. L. et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem. 271, 32529–32537 (1996).
Wenger, R. H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 (2002).
Buemi, M. et al. Erythropoietin and the brain: from neurodevelopment to neuroprotection. Clin. Sci. (Lond.) 103, 275–282 (2002).
Schmidt-Kastner, R. et al. Differential changes of bax, caspase-3 and p21 mRNA expression after transient focal brain ischemia in the rat. Brain Res. Mol. Brain Res. 79, 88–101 (2000).
van Lookeren Campagne, M. & Gill, R. Increased expression of cyclin G1 and p21WAF1/CIP1 in neurons following transient forebrain ischemia: comparison with early DNA damage. J. Neurosci. Res. 53, 279–296 (1998).
Sun, Y., Jin, K., Mao, X. O., Zhu, Y. & Greenberg, D. A. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl Acad. Sci. USA 98, 15306–15311 (2001).
Sun, Y. et al. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl Acad. Sci. USA 100, 3497–3500 (2003).
Ozaki, H. et al. Hypoxia inducible factor-1α is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest. Ophthalmol. Vis. Sci. 40, 182–189 (1999).
Junk, A. K. et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 99, 10659–10664 (2002).
Vinores, S. A. et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol. Histopathol. 12, 99–109 (1997).
Gidday, J. M. et al. Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxic preconditioning. J. Cereb. Blood Flow Metab. 19, 331–340 (1999).
Marber, M. S. & Yellon, D. M. Hypoxic preconditioning of ischaemic myocardium. Cardiovasc. Res. 26, 556–557 (1992).
Perez-Pinzon, M. A., Mumford, P. L., Rosenthal, M. & Sick, T. J. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience 75, 687–694 (1996).
Schurr, A., Payne, R. S., Tseng, M. T., Gozal, E. & Gozal, D. Excitotoxic preconditioning elicited by both glutamate and hypoxia and abolished by lactate transport inhibition in rat hippocampal slices. Neurosci. Lett. 307, 151–154 (2001).
Vannucci, R. C., Towfighi, J. & Vannucci, S. J. Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: pathologic and metabolic correlates. J. Neurochem. 71, 1215–1220 (1998).
Bernaudin, M. et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 22, 393–403 (2002).
Gidday, J. M., Fitzgibbons, J. C., Shah, A. R. & Park, T. S. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci. Lett. 168, 221–224 (1994).
Miller, B. A. et al. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport 12, 1663–1669 (2001). In this study, the authors report for the first time that hypoxic preconditioning protects against cerebral ischaemia in the adult brain as well as the neonatal brain.
Wick, A. et al. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J. Neurosci. 22, 6401–6407 (2002).
Bernaudin, M., Tang, Y., Reilly, M., Petit, E. & Sharp, F. R. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J. Biol. Chem. 277, 39728–39738 (2002). A genomic analysis revealing new genes that are regulated by hypoxic preconditioning and that might be implicated in brain oxygen sensing and in the brain's adaptation to subsequent ischaemia in the neonatal rat.
Jones, N. M. & Bergeron, M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J. Cereb. Blood Flow Metab. 21, 1105–1114 (2001).
Prass, K. et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J. Cereb. Blood Flow Metab. 22, 520–525 (2002).
Bernaudin, M. et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 19, 643–651 (1999).
Brines, M. L. et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc. Natl Acad. Sci. USA 97, 10526–10531 (2000).
Ehrenreich, H. et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8, 495–505 (2002).
Sakanaka, M. et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl Acad. Sci. USA 95, 4635–4640 (1998).
Lawrence, M. S., Ho, D. Y., Dash, R. & Sapolsky, R. M. Herpes simplex virus vectors overexpressing the glucose transporter gene protect against seizure-induced neuron loss. Proc. Natl Acad. Sci. USA 92, 7247–7251 (1995).
Rumora, L., Shaver, A., Zanic Grubisic, T. & Maysinger, D. MKP-1 as a target for pharmacological manipulations in PC12 cell survival. Neurochem. Int. 39, 25–32 (2001).
Yan, S. F. et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nature Med. 6, 1355–1361 (2000).
Beitner-Johnson, D. & Millhorn, D. E. Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. J. Biol. Chem. 273, 19834–19839 (1998).
Zaman, K. et al. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. 19, 9821–9830 (1999).
Blondeau, N., Widmann, C., Lazdunski, M. & Heurteaux, C. Activation of the nuclear factor-κB is a key event in brain tolerance. J. Neurosci. 21, 4668–4677 (2001).
Silverman, E. S. et al. Egr-1 and Sp1 interact functionally with the 5-lipoxygenase promoter and its naturally occurring mutants. Am. J. Respir. Cell Mol. Biol. 19, 316–323 (1998).
Murphy, B. J. et al. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res. 59, 1315–1322 (1999).
van Lookeren Campagne, M. et al. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. Proc. Natl Acad. Sci. USA 96, 12870–12875 (1999).
Sanchez-Elsner, T. et al. Synergistic cooperation between hypoxia and transforming growth factor-β pathways on human vascular endothelial growth factor gene expression. J. Biol. Chem. 276, 38527–38535 (2001).
Silins, G., Grimmond, S., Egerton, M. & Hayward, N. Analysis of the promoter region of the human VEGF-related factor gene. Biochem. Biophys. Res. Commun. 230, 413–418 (1997).
Hyder, S. M., Nawaz, Z., Chiappetta, C. & Stancel, G. M. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 60, 3183–3190 (2000).
Schipani, E. et al. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865–2876 (2001).
Simon, R. P. Hypoxia versus ischemia. Neurology 52, 7–8 (1999).
Tang, Y., Lu, A., Aronow, B. J., Wagner, K. R. & Sharp, F. R. Genomic responses of the brain to ischemic stroke, intracerebral haemorrhage, kainate seizures, hypoglycemia, and hypoxia. Eur. J. Neurosci. 15, 1937–1952 (2002).
Tang, Y., Nee, A. C., Lu, A., Ran, R. & Sharp, F. R. Blood genomic expression profile for neuronal injury. J. Cereb. Blood Flow Metab. 23, 310–319 (2003).
Englander, E. W., Greeley, G. H. Jr, Wang, G., Perez-Polo, J. R. & Lee, H. M. Hypoxia-induced mitochondrial and nuclear DNA damage in the rat brain. J. Neurosci. Res. 58, 262–269 (1999).
Clanton, T. L. & Klawitter, P. F. Adaptive responses of skeletal muscle to intermittent hypoxia: the known and the unknown. J. Appl. Physiol. 90, 2476–2487 (2001).
Reynolds, J. D. The management of retinopathy of prematurity. Paediatr. Drugs 3, 263–272 (2001).
Antonelli Incalzi, R. et al. Cognitive impairment in chronic obstructive pulmonary disease — a neuropsychological and spect study. J. Neurol. 250, 325–332 (2003).
Steen, R. G. et al. Cognitive impairment in children with hemoglobin SS sickle cell disease: relationship to MR imaging findings and hematocrit. Am. J. Neuroradiol. 24, 382–389 (2003).
Scortegagna, M. et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genet. 35, 331–340 (2003).
Cramer, T. & Johnson, R. S. A novel role for the hypoxia inducible transcription factor HIF-1α: critical regulation of inflammatory cell function. Cell Cycle 2, 192–193 (2003).
Piret, J. P., Mottet, D., Raes, M. & Michiels, C. Is HIF-1α a pro- or an anti-apoptotic protein? Biochem. Pharmacol. 64, 889–892 (2002).
Shaw, C. E. et al. Mutations in all five exons of SOD-1 may cause ALS. Ann. Neurol. 43, 390–394 (1998).
Naini, A. et al. Identification of a novel mutation in Cu/Zn superoxide dismutase gene associated with familial amyotrophic lateral sclerosis. J. Neurol. Sci. 198, 17–19 (2002).
Oosthuyse, B. et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nature Genet. 28, 131–138 (2001).
Nygren, I., Larsson, A., Johansson, A. & Askmark, H. VEGF is increased in serum but not in spinal cord from patients with amyotrophic lateral sclerosis. Neuroreport 13, 2199–2201 (2002).
Millhorn, D. E. et al. Regulation of gene expression for tyrosine hydroxylase in oxygen sensitive cells by hypoxia. Kidney Int. 51, 527–535 (1997).
Maher, E. R. & Kaelin, W. G. Jr. von Hippel-Lindau disease. Medicine (Baltimore) 76, 381–391 (1997).
Ivan, M. & Kaelin, W. G. Jr. The von Hippel-Lindau tumor suppressor protein. Curr. Opin. Genet. Dev. 11, 27–34 (2001).
Ratcliffe, P. J. New insights into an enigmatic tumour suppressor. Nature Cell Biol. 5, 7–8 (2003).
Kung, A. L., Wang, S., Klco, J. M., Kaelin, W. G. & Livingston, D. M. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nature Med. 6, 1335–1340 (2000). This study gives clear evidence that disrupting the interaction of HIF1 and p300/CBP has an anti-tumoral effect.
Birner, P. et al. Expression of hypoxia-inducible factor-1α in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer 92, 165–171 (2001).
Sondergaard, K. L., Hilton, D. A., Penney, M., Ollerenshaw, M. & Demaine, A. G. Expression of hypoxia-inducible factor 1α in tumours of patients with glioblastoma. Neuropathol. Appl. Neurobiol. 28, 210–217 (2002).
Khatua, S. et al. Overexpression of the EGFR/FKBP12/HIF-2α pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 63, 1865–1870 (2003).
Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 29, 10–14 (2002).
An, W. G. et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392, 405–408 (1998).
Brusselmans, K. et al. Hypoxia-inducible factor-2α (HIF-2α) is involved in the apoptotic response to hypoglycemia but not to hypoxia. J. Biol. Chem. 276, 39192–39196 (2001).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Halterman, M. W., Miller, C. C. & Federoff, H. J. Hypoxia-inducible factor-1α mediates hypoxia-induced delayed neuronal death that involves p53. J. Neurosci. 19, 6818–6824 (1999).
Goda, N. et al. Hypoxia-inducible factor 1α is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 23, 359–369 (2003).
Acknowledgements
We would like to thank J. Gidday, J. LaManna, E. Petit and S. Vannucci for their comments on the manuscript. We are supported by NINDS/National Institutes of Health grants, the CNRS and the University of Caen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- BARORECEPTORS
-
Cells, located in the arteries, that monitor blood pressure.
- BASIC HELIX–LOOP–HELIX
-
(bHLH). A structural motif present in many transcription factors that is characterized by two α-helices separated by a loop. The helices mediate dimerization, and the adjacent basic region is required for DNA binding.
- NORMOXIC
-
At or containing a normal level of oxygen.
- EPENDYMAL CELLS
-
A layer of cells that line the large fluid-filled cavities in the brain, the ventricles.
- PURKINJE CELLS
-
Inhibitory neurons in the cerebellum that use GABA (γ-aminobutyric acid) as their neurotransmitter. Their cell bodies are situated beneath the molecular layer, and their dendrites branch extensively in this layer. Their axons project into the underlying white matter, and they provide the only output from the cerebellar cortex.
- HYDROCEPHALUS
-
A condition, marked by an expansion of the cerebral ventricles and a compression of neural structures, that is caused by a block in the flow of cerebral spinal fluid or by its overproduction.
- MYELOID
-
In the haematopoietic system, myeloid refers to granulocytes and monocytes/macrophages and their precursors.
- ANGIOGENESIS
-
The formation of blood vessels, such as occurs during embryogenesis, tissue repair or tumorigenesis.
- PROTEASOMAL
-
A term that refers to a protein complex that is responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- ERYTHROPOIETIN
-
(EPO). A renal hormone that is induced by anaemia and that activates haemoglobin synthesis by bone-marrow red-cell precursors.
- ANTISENSE
-
Oligonucleotides with a sequence that is complementary to the mRNA of a given molecule can be used to block its translation. The subsequent temporary elimination of the protein of interest often provides useful information on its biological function.
- SMALL INHIBITORY RNA
-
A small RNA molecule that interferes with normal RNA processing, causing rapid degradation of the endogenous RNA and thereby precluding translation. This provides a simple way of studying the effects of the absence of a gene product in simple organisms and in cells.
- SICKLE CELL DISEASE
-
A red blood cell disorder that is caused by a point mutation in the gene that codes for the oxygen-transport protein haemoglobin. In the homozygous state, this mutation causes haemoglobin molecules to aggregate and form long chains when they release oxygen, causing the red blood cell to adopt the characteristic sickle shape. These sickle-shaped cells can cause blockages in blood vessels. There is also an increase in red blood cell destruction, leading to anaemia.
Rights and permissions
About this article
Cite this article
Sharp, F., Bernaudin, M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5, 437–448 (2004). https://doi.org/10.1038/nrn1408
Issue Date:
DOI: https://doi.org/10.1038/nrn1408
This article is cited by
-
Neuronal Gtf2i deletion alters mitochondrial and autophagic properties
Communications Biology (2023)
-
NSD1 promotes esophageal cancer tumorigenesis via HIF1α signaling
Cell Biology and Toxicology (2023)
-
An overview of hyperbaric oxygen preconditioning against ischemic stroke
Metabolic Brain Disease (2023)
-
Genetic mutations affecting mitochondrial function in cancer drug resistance
Genes & Genomics (2023)
-
The role of brain inflammation and abnormal brain oxygen homeostasis in the development of hepatic encephalopathy
Metabolic Brain Disease (2023)