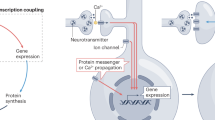
Key Points
-
Various forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), are initiated through synapse-specific increases in Ca2+ that are generated through the activation of voltage-sensitive Ca2+ channels or NMDA (N-methyl-D-aspartate) receptors.
-
Many of the effects of Ca2+ on synaptic efficiency are mediated through the Ca2+-binding protein calmodulin (CaM), which mediates Ca2+ stimulation of several enzymes that are crucial for synaptic plasticity. The affinity of CaM for most of these proteins is increased when the concentration of free Ca2+ increases. Therefore, CaM mediates activity-dependent stimulation of a family of enzymes that are important for synaptic plasticity.
-
The unique regulatory properties of CaM allow various target enzymes with different Ca2+ sensitivities to be stimulated and to show varying degrees of positive cooperativity.
-
Several novel CaM-binding proteins, including neuromodulin and neurogranin, localize and control the levels of free CaM in neurons. These IQ domain-containing proteins bind CaM with higher affinity in the absence of Ca2+ compared with in the presence of Ca2+. Furthermore, neuromodulin and neurogranin can be phosphorylated at sites within an IQ domain, which is also the CaM-binding domain. Consequently, activity-dependent Ca2+ increases the release of CaM from neuromodulin and neurogranin.
-
Calcium/calmodulin-dependent protein kinase II (CaMKII), a CaM-stimulated kinase, has a crucial role in several forms of LTP. CaMKII might contribute to LTP by augmenting the activity of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors and stimulating dendritic translation. CaMKIV phosphorylates the CREB (cAMP responsive element (CRE)-binding protein) binding protein (CBP), which is a necessary event for Ca2+ stimulation of CRE-mediated transcription and heterosynaptic late-phase LTP.
-
Calcineurin, a CaM-stimulated protein phosphatase, is thought to catalyse the dephosphorylation of AMPA receptors, an important event in LTD.
-
The CaM-stimulated adenylyl cyclases AC1 and AC8 are thought to contribute to LTP by coupling activity-dependent Ca2+ increases to elevated cAMP. This cAMP signal activates protein kinase A (PKA), which phosphorylates and enhances the activity of AMPA. In addition, cAMP increases ERK/MAPK activity, a crucial activity for L-LTP.
Abstract
Excitatory synapses in the brain show several forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD), which are initiated by increases in intracellular Ca2+ that are generated through NMDA (N-methyl-D-aspartate) receptors or voltage-sensitive Ca2+ channels. LTP depends on the coordinated regulation of an ensemble of enzymes, including Ca2+/calmodulin-dependent protein kinase II, adenylyl cyclase 1 and 8, and calcineurin, all of which are stimulated by calmodulin, a Ca2+-binding protein. In this review, we discuss the hypothesis that calmodulin is a central integrator of synaptic plasticity and that its unique regulatory properties allow the integration of several forms of signal transduction that are required for LTP and LTD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Malenka, R. C. & Bear, M. F. LTP and LTD; an embarrassment of riches. Neuron 44, 5–21 (2004).
Matynia, A., Kushner, S. A. & Silva, A. J. Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu. Rev. Genet. 36, 687–720 (2002).
Cimler, B. M., Andreasen, T. J., Andreasen, K. I. & Storm, D. R. P-57 is a neural specific calmodulin-binding protein. J. Biol. Chem. 260, 10784–10788 (1985).
LaPorte, D. C., Wierman, B. M. & Storm, D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry 19, 3814–3819 (1980).
Babu, Y. S., Bugg, C. E. & Cook, W. J. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 204, 191–204 (1988).
Chapman, E. R., Alexander, K., Vorherr, T., Carafoli, E. & Storm, D. R. Fluorescence energy transfer analysis of calmodulin-peptide complexes. Biochemistry 31, 12819–12825 (1992).
Levin, R. M. & Weiss, B. Specificity of the binding of trifluoperazine to the calcium-dependent activator of phosphodiesterase and to a series of other calcium-binding proteins. Biochim. Biophys. Acta 540, 197–204 (1978).
Weiss, B., Prozialeck, W., Cimino, M., Barnette, M. S. & Wallace, T. L. Pharmacological regulation of calmodulin. Ann. NY Acad. Sci. 356, 319–345 (1980).
Olwin, B. B., Edelman, A. M., Krebs, E. G. & Storm, D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J. Biol. Chem. 259, 10949–10955 (1984).
Olwin, B. B. & Storm, D. R. Binding of calcium to calmodulin–protein complexes. Biochemistry 24, 8081–8086 (1985).
Andreasen, T. J., Luetje, C. W., Heideman, W. & Storm, D. R. Purification of a novel calmodulin binding protein from bovine cerebral cortex membranes. Biochemistry 22, 4615–4618 (1983).
Alexander, K. A., Wakim, B. T., Doyle, G. S., Walsh, K. A. & Storm, D. R. Identification and characterization of the calmodulin-binding domain of neuromodulin, a neurospecific calmodulin-binding protein. J. Biol. Chem. 263, 7544–7549 (1988). References 11 and 12 were the first papers to report that neuromodulin is a CaM–binding protein with higher affinity for CaM in the absence of Ca2+ than in its presence.
Apel, E. D., Byford, M. F., Au, D., Walsh, K. A. & Storm, D. R. Identification of the protein kinase C phosphorylation site in neuromodulin. Biochemistry 29, 2330–2335 (1990).
Chao, S., Benowitz, L. I., Krainc, D. & Irwin, N. Use of a two-hybrid system to investigate molecular interactions of GAP-43. Brain Res. Mol. Brain Res. 40, 195–202 (1996).
Gamby, C., Waage, M. C., Allen, R. G. & Baizer, L. Analysis of the role of calmodulin binding and sequestration in neuromodulin (GAP-43) function. J. Biol. Chem. 271, 26698–26705 (1996).
Nelson, R. B. & Routtenberg, A. Characterization of protein F1 (47 kDa, 4.5 pI): a kinase C substrate directly related to neural plasticity. Exp. Neurol. 89, 213–224 (1985).
Kleschevnikov, A. M. & Routtenberg, A. PKC activation rescues LTP from NMDA receptor blockade. Hippocampus 11, 168–175 (2001).
Hulo, S., Alberi, S., Laux, T., Muller, D. & Caroni, P. A point mutant of GAP-43 induces enhanced short-term and long-term hippocampal plasticity. Eur. J. Neurosci. 15, 1976–1982 (2002). Showed that overexpression of a mutated form of neuromodulin that mimics the protein phosphorylated at Ser41 enhances CA1 LTP. This paper supports the hypothesis that phosphorylation of neuromodulin at Ser41 by PKC releases CaM and enhances synaptic efficacy.
Represa, A., Deloulme, J. C., Sensenbrenner, M., Ben-Ari, Y. & Baudier, J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J. Neurosci. 10, 3782–3792 (1990).
Baudier, J., Deloulme, J. C., Van-Dorsselaer, A., Black, D. & Matthes, H. W. Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin binding domain. J. Biol. Chem. 266, 229–237 (1991).
Prichard, L., Deloulme, J. C. & Storm, D. R. Interactions between neurogranin and calmodulin in vivo. J. Biol. Chem. 274, 7689–7694 (1999).
Krucker, T. et al. Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus. J. Neurosci. 22, 5525–5535 (2002).
Gerendasy, D. D. & Sutcliffe, J. G. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Mol. Neurobiol. 15, 131–163 (1997).
Rakhilin, S. V. et al. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science 306, 698–701 (2004).
Hudmon, A. & Schulman, H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71, 473–510 (2002).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Rev. Neurosci. 3, 175–190 (2002).
Fink, C. C. & Meyer, T. Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr. Opin. Neurobiol. 12, 293–299 (2002).
Colbran, R. J. & Brown, A. M. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 14, 318–327 (2004).
DeKoninck, P. & Schulman, H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230 (1998).
Torok, K., Tzortzopoulos, A., Grabarek, Z., Best, S. L. & Thorogate, R. Dual effect of ATP in the activation mechanism of brain Ca2+/calmodulin-dependent protein kinase II by Ca2+/calmodulin. Biochemistry 40, 14878–14890 (2001).
Shen, K. & Meyer, T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284, 162–166 (1999).
Bayer, K. U., De Koninck, P., Leonard, A. S., Hell, J. W. & Schulman, H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411, 801–805 (2001). The first evidence that the interaction between NMDA receptors and CaMKII leads to the trapping of CaM, an interaction that might decrease the downregulation of NMDA receptor activity.
Miller, S. G. & Kennedy, M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44, 861–870 (1986).
Malinow, R., Schulman, H. & Tsien, R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866 (1989).
Malenka, R. C. et al. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340, 554–557 (1989). References 34 and 35 were the first studies to use enzyme inhibitors to implicate CaMKII in LTP.
Fukunaga, K., Stoppini, L., Miyamoto, E. & Muller, D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 268, 7863–7867 (1993).
McGlade-McCulloh, E., Yamamoto, H., Tan, S. E., Brickey, D. A. & Soderling, T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362, 640–642 (1993).
Lledo, P. M. et al. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc. Natl Acad. Sci. USA 92, 11175–11179 (1995).
Silva, A. J., Stevens, C. F., Tonegawa, S. & Wang, Y. Deficient hippocampal long-term potentiation in α-calmodulin kinase II mutant mice. Science 257, 201–206 (1992). References 38 and 39 were the first studies to use gene disruption technology to implicate CaMKII in LTP and memory.
Silva, A. J., Paylor, R., Wehner, J. M. & Tonegawa, S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science 257, 206–211 (1992).
Giese, K. P., Fedorov, N. B., Filipkowski, R. K. & Silva, A. J. Autophosphorylation at Thr(286) of the α calcium-calmodulin kinase II in LTP and learning. Science 279, 870–873 (1998).
Kauer, J. A., Malenka, R. C. & Nicoll, R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1, 911–917 (1988).
Muller, D. & Lynch, G. Long-term potentiation differentially affects two components of synaptic responses in hippocampus. Proc. Natl Acad. Sci. USA 85, 9346–9350 (1988).
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Nusser, Z. et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21, 545–559 (1998).
Barria, A., Muller, D., Derkach, V., Griffith, L. C. & Soderling, T. R. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042–2045 (1997).
Mammen, A. L., Kameyama, K., Roche, K. W. & Huganir, R. L. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 272, 32528–32533 (1997).
Wu, G. Y., Malinow, R. & Cline, H. T. Maturation of a central glutamatergic synapse. Science 274, 972–976 (1996).
Lisman, J. E. & Zhabotinsky, A. M. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31, 191–201 (2001). Presents an interesting model that describes how CaMKII and PP1 can function as a bistable switch that is activated during LTP.
Lee, H. K. et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 (2003). The first study to show that phosphorylation of AMPA receptors at both phosphorylation sites is required for LTP. Transgenic mice with knock-in mutations in the AMPA phosphorylation sites show defects in LTP and LTD.
Poser, S. & Storm, D. R. Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. Int. J. Dev. Neurosci. 19, 387–394 (2001).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004). Evidence that CaMKII activity is required for the glutamate-induced enlargement of stimulated spines.
Kim, C. H. & Lisman, J. E. A role of actin filament in synaptic transmission and long-term potentiation. J. Neurosci. 19, 4314–4424 (1999).
Krucker, T., Siggins, G. R. & Halpain, S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc. Natl Acad. Sci. USA 97, 6856–6861 (2000).
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl Acad. Sci. USA 99, 13902–13907 (2002).
Tiedge, H. & Brosius, J. Translational machinery in dendrites of hippocampal neurons in culture. J. Neurosci. 16, 7171–7181 (1996).
Steward, O. & Schuman, E. M. Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci. 24, 299–325 (2001).
Frey, U. & Morris, R. G. M. Synaptic tagging and long-term potentiation. Nature 385, 533–536 (1997).
Mayford, M., Baranes, D., Podsypanina, K. & Kandel, E. R. The 3′-untranslated region of CaMKII α is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc. Natl Acad. Sci. USA 93, 13250–13255 (1996).
Thomas, K. L., Laroche, S., Errington, M. L., Bliss, T. V. & Hunt, S. P. Spatial and temporal changes in signal transduction pathways during LTP. Neuron 13, 737–745 (1994).
Ouyang, Y., Rosenstein, A., Kreiman, G., Schuman, E. M. & Kennedy, M. B. Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J. Neurosci. 19, 7823–7833 (1999).
Miller, S. et al. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36, 507–519 (2002). In this study, the CaMKII gene was mutated to disrupt the dendritic localization signal in the mRNA with the protein-coding region intact. Removal of dendritic mRNA reduced CaMKII in the PSDs and decreased L-LTP.
Atkins, C. M., Nozaki, N., Shigeri, Y. & Soderling, T. R. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 24, 5193–5201 (2004).
Ninan, I. & Arancio, O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron 42, 129–141 (2004).
Greengard, P., Valtorta, F., Czernik, A. J. & Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259, 780–785 (1993).
Chi, P., Greengard, P. & Ryan, T. A. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 38, 69–78 (2003).
Bos, J. L. Epac: a new cAMP target and new avenues in cAMP research. Nature Rev. Mol. Cell Biol. 4, 733–738 (2003).
English, J. D. & Sweatt, D. J. Activation of p42 MAP kinase in hippocampal long term potentiation. J. Biol. Chem. 271, 24329–24332 (1996).
Impey, S. et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21, 869–883 (1998).
Frey, U., Reymann, K. G. & Matthies, H. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci. Lett. 129, 111–114 (1991).
Frey, U., Huang, Y. Y. & Kandel, E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260, 1661–1664 (1993).
Impey, S. et al. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron 16, 973–982 (1996).
Abel, T., Nguyen, P. V., Barad, M., Deuel, T. A. S. & Kandel, E. R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626 (1997).
Nguyen, P. V. & Kandel, E. R. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J. Neurosci. 16, 3189–3198 (1996).
Huang, Y. Y., Pittenger, C. & Kandel, E. R. A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing. Proc. Natl Acad. Sci. USA 101, 859–864 (2004). This paper reports that heterosynaptically induced LTP requires PKA activity as well as protein synthesis. Transgenic mice that express a dominant-negative CREB inhibitor in the hippocampus have impaired heterosynaptic L-LTP.
Xia, Z. G., Refsdal, C. D., Merchant, K. M., Dorsa, D. M. & Storm, D. R. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron 6, 431–443 (1991).
Cali, J. J., Zwaagstra, C., Mons, N., Cooper, D. M. F. & Krupinski, J. Type VIII adenylyl cyclase: a Ca2+/calmodulin stimulated enzyme expressed in discrete regions of rat brain. J. Biol. Chem. 269, 12190–12196 (1994).
Stanton, P. K. & Sarvey, J. M. The effect of high-frequency electrical stimulation and norepinephrine on cyclic AMP levels in normal versus norepinephrine-depleted rat hippocampal slices. Brain Res. 358, 343–348 (1985).
Chetkovich, D. M. & Sweatt, J. D. NMDA receptor activation increases cAMP in area CA1 of the hippocampus via Ca2+/calmodulin stimulation of adenylyl cyclase. J. Neurochem. 61, 1933–1942 (1993).
Huang, Y. Y. et al. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell 83, 1211–1222 (1995).
Brandon, E. P. et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RIβ subunit of cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 92, 8851–8855 (1995).
Pineda, V. V. et al. Removal of Giα1 constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron 41, 153–163 (2004).
Blitzer, R. D. et al. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science 280, 1940–1943 (1998).
Wang, H. & Storm, D. R. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 63, 463–468 (2003).
Xia, Z., Choi, E. J., Wang, F., Blazynski, C. & Storm, D. R. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J. Neurochem. 60, 305–311 (1993).
Wu, Z. L. et al. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl Acad. Sci. USA 92, 220–224 (1995).
Otmakhova, N. A., Otmakhov, N., Mortenson, L. H. & Lisman, J. E. Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 20, 4446–4451 (2000). References 87 and 88 provide the first evidence that CaM-stimulated adenylyl cyclases and cAMP signalling are required for E-LTP.
Villacres, E. C., Wong, S. T., Chavkin, C. & Storm, D. R. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J. Neurosci. 18, 3186–3194 (1998).
Storm, D. R., Hansel, C., Hacker, B., Parent, A. & Linden, D. J. Impaired cerebellar LTP in type I adenylyl cyclase mutant mice. Neuron 20, 1199–1210 (1998). References 89 and 90 provide evidence that CaM-stimulated adenylyl cyclases are required for presynaptic LTP.
Wang, H. et al. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J. Neurosci. 23, 9710–9718 (2003).
Weisskopf, M. G., Castillo, P. E., Zalutsky, R. A. & Nicoll, R. A. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265, 1878–1882 (1994).
Salin, P. A., Malenka, R. C. & Nicoll, R. A. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16, 797–803 (1996).
Castillo, P. E. et al. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388, 590–593 (1997).
Wong, S. T. et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus dependent long-term memory and late-phase LTP. Neuron 23, 787–798 (1999). The first evidence that CaM-stimulated adenylyl cyclases are required for L-LTP and LTM.
Wang, H., Ferguson, G. D., Pineda, V. V., Cundiff, P. E. & Storm, D. R. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nature Neurosci. 7, 635–642 (2004).
Wang, J. H. & Desai, R. A brain protein and its effect on the Ca2+- and protein modulator-activated cyclic nucleotide phosphodiesterase. Biochem. Biophys. Res. Commun. 72, 926–937 (1976).
Klee, C. B., Crouch, T. H. & Krinks, M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl Acad. Sci. USA 76, 6270–6273 (1979).
Takaishi, T., Saito, N., Kuno, T. & Tanaka, C. Differential distribution of the mRNA encoding two isoforms of the catalytic subunit of calcineurin in the rat brain. Biochem. Biophys. Res. Commun. 174, 393–398 (1991).
Kuno, T. et al. Distinct cellular expression of calcineurin A α and A β in rat brain. J. Neurochem. 58, 1643–1651 (1992).
Liu, Y. C. & Storm, D. R. Dephosphorylation of neuromodulin by calcineurin. J. Biol. Chem. 264, 12800–12804 (1989).
Seki, K., Chen, H. C. & Huang, K. P. Dephosphorylation of protein kinase C substrates, neurogranin, neuromodulin, and MARCKS, by calcineurin and protein phosphatases 1 and 2A. Arch. Biochem. Biophys. 316, 673–679 (1995).
Mulkey, R. M., Endo, S., Shenolikar, S. & Malenka, R. C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369, 486–488 (1994).
Malleret, G. et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104, 675–686 (2001). Using a doxycycline-dependent rtTA system to express a calcineurin inhibitor in the mouse brain, the authors showed that a reduction in calcineurin activity enhances LTP.
Migaud, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 (1998).
Dudek, S. M. & Bear, M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl Acad. Sci. USA 89, 4363–4367 (1992).
Mulkey, R. M., Herron, C. E. & Malenka, R. C. An essential role for protein phosphatases in hippocampal long-term depression. Science 261, 1051–1055 (1993).
Lisman, J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl Acad. Sci. USA 86, 9574–9578 (1989).
Banke, T. G. et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20, 89–102 (2000).
Lee, H. K., Barbarosie, M., Kameyama, K., Bear, M. F. & Huganir, R. L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 (2000).
Shi, J., Townsend, M. & Constantine-Paton, M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron 28, 103–114 (2000).
Beattie, E. C. et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neurosci. 3, 1291–1300 (2000).
Liu, Y. C. & Storm, D. R. Regulation of free calmodulin levels by neuromodulin: neuron growth and regeneration. Trends Pharmacol. Sci. 11, 107–111 (1990).
Ramakers, G. M., Heinen, K., Gispen, W. H. & de Graan, P. N. Long term depression in the CA1 field is associated with a transient decrease in pre- and postsynaptic PKC substrate phosphorylation. J. Biol. Chem. 275, 28682–28687 (2000).
Frey, U., Krug, M., Reymann, K. G. & Matthies, H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 452, 57–65 (1988).
Tully, T., Preat, T., Boynton, S. C. & Del Vecchio, M. Genetic disection of consolidated memory in Drosophila. Cell 79, 35–47 (1994).
Flexner, L. B. & Flexner, J. B. Effect of acetoxycycloheximide and of an acetoxycycloheximide-puromycin mixture on cerebral protein synthesis and memory in mice. Proc. Natl Acad. Sci. USA 55, 369–374 (1966).
Squire, L. R. & Barondes, S. H. Memory impairment during prolonged training in mice given inhibitors of cerebral protein synthesis. Brain Res. 56, 215–225 (1973).
Crow, T. & Forrester, J. Inhibition of protein synthesis blocks long-term enhancement of generator potentials produced by one-trial in vivo conditioning in Hermissenda. Proc. Natl Acad. Sci. USA 87, 4490–4494 (1990).
Dash, P. K., Hochner, B. & Kandel, E. R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345, 718–721 (1990).
Martin, K. C. et al. Synapse-specific, long-form facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91, 927–938 (1997).
Yin, J. C. P. et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79, 49–58 (1994).
Yin, J. C., Del Vecchio, M., Zhou, H. & Tully, T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115 (1995).
Bourtchuladze, R. et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79, 59–68 (1994).
Impey, S. et al. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nature Neurosci. 1, 595–601 (1998).
Athos, J., Impey, S., Pineda, V. V., Chen, X. & Storm, D. R. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nature Neurosci. 5, 1119–1120 (2002).
Pittenger, C. et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34, 447–462 (2002).
Barco, A., Alarcon, J. M. & Kandel, E. R. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108, 689–703 (2002). Direct evidence using transgenic mouse technology that CREB can regulate L-LTP.
Bito, H., Deisseroth, K. & Tsien, R. W. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 (1996).
Wu, G. Y., Deisseroth, K. & Tsien, R. W. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl Acad. Sci. USA 98, 2808–2813 (2001).
Xing, J., Ginty, D. D. & Greenberg, M. E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273, 959–963 (1996).
Deak, M., Clifton, A. D., Lucocq, J. M. & Alessi, D. R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 (1998).
English, J. D. & Sweatt, J. D. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J. Biol. Chem. 272, 19103–19106 (1997). The first evidence that signalling through the ERK/MAPK pathway is required for LTP.
Atkins, C. M., Selcher, J. C., Petraitis, J. J., Trzaskos, J. M. & Sweatt, J. D. The MAPK cascade is required for mammalian associative learning. Nature Neurosci. 1, 602–609 (1998).
Deisseroth, K., Bito, H. & Tsien, R. W. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16, 89–101 (1996).
Fields, R. D., Eshete, F., Stevens, B. & Itoh, K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+ cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J. Neurosci. 17, 7252–7266 (1997).
Liu, F. C. & Graybiel, A. M. Spatiotemporal dynamics of CREB phosphorylation: transient versus sustained phosphorylation in the developing striatum. Neuron 17, 1133–1144 (1996).
Impey, S. et al. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron 34, 235–244 (2002).
Ebinu, J. O. et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 (1998).
de Rooij, J. et al. Epac is a Rap1 guanine-nucleotide exchange factor directly activated by cAMP. Nature 396, 474–477 (1998).
Keller, C. H., Olwin, B. B., LaPorte, D. C. & Storm, D. R. Determination of the free-energy coupling for binding of calcium ions and troponin I to calmodulin. Biochemistry 21, 156–162 (1982).
Acknowledgements
Both authors are supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- FLUORESCENCE RESONANCE ENERGY TRANSFER
-
(FRET). A spectroscopic technique that is based on the transfer of energy from the excited state of a donor moiety to an acceptor. The transfer efficiency depends on the distance between the donor and the acceptor. FRET is often used to estimate distances between macromolecular sites in the 20–100-Å range, or to study interactions between macromolecules in vivo.
- DISSOCIATION CONSTANT
-
A measure to describe the strength of binding (or affinity) between receptors and their ligands.
- CaM–SEPHAROSE AFFINITY CHROMATOGRAPHY
-
An affinity column for the purification of CaM-binding proteins comprised of CaM covalently attached to Sepharose. Most CaM-binding proteins bind to CaM–Sepharose in the presence of Ca2+. Neuromodulin binds to the column in the absence of Ca2+ and is eluted from the column with buffers that contain Ca2+.
- IQ DOMAIN
-
A small structural domain, originally identified in neuromodulin, that mediates interactions with CaM. The motif only loosely defines the amino acid sequence at 5 of 11 possible residues. Different IQ domains bind CaM at varying intracellular Ca2+ concentrations or independently of Ca2+.
- TETANIC STIMULATION
-
A train of stimuli that causes brief, high-frequency activation of afferent axons. In LTP experiments, a 1-s train of pulses delivered at a frequency of 100 Hz is commonly used to potentiate transmission.
- SCHAFFER COLLATERALS
-
Axons of the CA3 pyramidal cells of the hippocampus that form synapses with the apical dendrites of CA1 neurons.
- PAIRING PROTOCOL
-
If a cell is artificially depolarized while low-frequency stimulation is delivered, synaptic transmission will be potentiated because the depolarization relieves the Mg2+-dependent block of NMDA receptors.
- SYNAPTIC TAGGING
-
A mechanism by which activated synapses are tagged to distinguish them from other synapses on the same neuron that have not been activated. The mechanism for synaptic tagging is not known, but might be due to increases in dendritic translation.
- LTD/LTP THRESHOLD
-
The frequency at which an LTD response is converted to LTP.
Rights and permissions
About this article
Cite this article
Xia, Z., Storm, D. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 6, 267–276 (2005). https://doi.org/10.1038/nrn1647
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn1647
This article is cited by
-
Monitoring synaptic pathology in Alzheimer’s disease through fluid and PET imaging biomarkers: a comprehensive review and future perspectives
Molecular Psychiatry (2024)
-
Immobilization of azide-functionalized proteins to micro- and nanoparticles directly from cell lysate
Microchimica Acta (2024)
-
The release of inhibition model reproduces kinetics and plasticity of neurotransmitter release in central synapses
Communications Biology (2023)
-
Preclinical models of treatment-resistant depression: challenges and perspectives
Pharmacological Reports (2023)
-
Multi-proteomic Analysis Revealed Distinct Protein Profiles in Cerebrospinal Fluid of Patients Between Anti-NMDAR Encephalitis NORSE and Cryptogenic NORSE
Molecular Neurobiology (2023)