Abstract
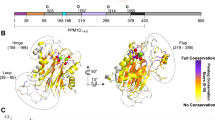
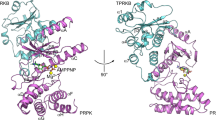
MEK1 and MEK2 are closely related, dual-specificity tyrosine/threonine protein kinases found in the Ras/Raf/MEK/ERK mitogen-activated protein kinase (MAPK) signaling pathway. Approximately 30% of all human cancers have a constitutively activated MAPK pathway, and constitutive activation of MEK1 results in cellular transformation. Here we present the X-ray structures of human MEK1 and MEK2, each determined as a ternary complex with MgATP and an inhibitor to a resolution of 2.4 Å and 3.2 Å, respectively. The structures reveal that MEK1 and MEK2 each have a unique inhibitor-binding pocket adjacent to the MgATP-binding site. The presence of the potent inhibitor induces several conformational changes in the unphosphorylated MEK1 and MEK2 enzymes that lock them into a closed but catalytically inactive species. Thus, the structures reported here reveal a novel, noncompetitive mechanism for protein kinase inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ahn, N.G. et al. Multiple components in an epidermal growth factor–stimulated protein kinase cascade: in vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J. Biol. Chem. 266, 4220–4227 (1991).
Zheng, C-F. & Guan, K. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J. Biol. Chem. 268, 11435–11439 (1993).
Dhanasekaran, N. & Reddy, P. Signaling by dual-specificity kinases. Oncogene 17, 1447–1455 (1998).
Kolch, W. Ras/Raf signaling and emerging pharmacotherapeutic targets. Expert Opin. Pharmacother. 3, 709–718 (2002).
Schaeffer, H.J. & Weber, M.J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19, 2435–2444 (1999).
Lewis, T.S., Shapiro P.S. & Ahn, N.G. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74, 49–139 (1998).
Alessi, D.R. et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74 Raf-1. EMBO J. 13, 1610–1619 (1994).
Herrera, R. & Sebolt-Leopold, J.S. Unravelling the complexities of the Raf/MAP kinase pathway for pharmacological intervention. Trends Mol. Med. 8, S27–S31 (2002).
Kyriakis, J.M. & Avruch, J. Mammalian mitogen-activated signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2002).
Dudley, D.T., Pang, L., Decker, S.J., Bridges, A.J. & Saltiel, A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92, 7686–7689 (1995).
Sebolt-Leopold, J.S. et al. Blockade of the MAP kinase pathway suppresses growth of colon tumours in vivo. Nat. Med. 5, 810–816 (1999).
Delaney, A.M., Printen, J.A., Chen, H., Fauman, E.B. & Dudley, D.T. Identification of a novel mitogen-activated protein kinase kinase activation domain recognized by the inhibitor PD184352. Mol. Cell. Biol. 22, 7593–7602 (2002).
Dang, A., Frost, J.A. & Cobb, M.H. The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273, 19909–19913 (1998).
Hanks, S.K. & Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9, 576–596 (1995).
Zheng, J. et al. 2.2 Å refined crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MnATP and a peptide inhibitor. Acta Crystallogr. D 49, 362–365 (1993).
Hubbard, S.R. et al. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 16, 5572–5581 (1997).
Janin, J. & Chothia, C. The structure of protein-protein recognition sites. J. Biol. Chem. 265, 16027–16030 (1990).
Mizuguchi, K., Deane, C.M., Blundell, T.L., Johnson, M.S. & Overington, J.P. JOY: protein sequence-structure representation and analysis. Bioinformatics 14, 617–623 (1998).
De Azevedo, W.F. et al. Structural basis for specificity and potency of a flavonoid inhibitor of human Cdk2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 93, 2735–2740 (1996).
Pargellis, C. et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat. Struct. Biol. 9, 268–272 (2002).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289, 1938–1942 (2000).
De Bondt, H. et al. Crystal structure of cyclin-dependent kinase 2. Nature 363, 595–602 (1993).
Xu, W. et al. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385, 595–602 (1997).
Sicheri, F. et al. Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 (1997).
Parang, K. & Cole, P.A. Designing bisubstrate analog inhibitors for protein kinases. Pharmacol. Ther. 93, 145–157 (2002).
Manning, G. et al. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Davies, S.P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Mody, N., Leitch, J., Armstrong, C., Dixon, J. & Cohen, P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 502, 21–24 (2001).
Kailesh, G. et al. Negative regulation of MAPKK by phosphorylation of a conserved serine residue equivalent to Ser212 of MEK1 J. Biol. Chem. 278, 8118–8125 (2003).
Zhang, J., Zhou, B., Zheng, C-F. & Zhang, Z-Y. A bipartite mechanism for ERK2 recognition by its cognate regulators and substrates. J. Biol. Chem. 278, 29901–29912 (2003).
Robinson, F.L., Whitehurst, A.W., Raman, M. & Cobb, M.H. Identification of novel point mutations in ERK2 that selectively disrupt binding to MEK1. J. Biol. Chem. 277, 14848–14852 (2002).
Wilsbacher, J.L., Goldsmith, E.J. & Cobb, M.H. Phosphorylation of MAP kinase by MAP/ERK involves multiple regions of MAP kinases. J. Biol. Chem. 274, 16988–16994 (1999).
Yang, C. & Pflugrath, J.W. Applications of anomalous scattering from S atoms for improved phasing of protein diffraction data collected at Cu K wavelength. Acta Crystallogr. D 57, 1480–1490 (2001).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Brunger, A.T. et al. Crystallography & NMR system (CNS): a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997).
Collaborative Computational Project, Number 4. CCP4 Suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Vagin, A.A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Laue, T.M., Shah, B.D., Ridgeway, T.M. & Pelletier, S.L. Computer-aided interpretation of analytical sedimentation data for proteins. In Analytical Ultracentrifugation in Biochemistry and Polymer Science (eds. Harding, S.E., Rowe, A.J. & Horton, J.C.) 90–125 (The Royal Society of Chemistry, Cambridge, 1992).
Carson, M. Ribbon models of macromolecules. J. Mol. Graphics 5, 103–106 (1987).
Acknowledgements
We thank M. Dumond for assistance in the crystallization of MEK1, and N. Ahn, B. Lunney, B. Finzel and N. Chirgadze for their thoughtful review of the manuscript. Use of the IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Illinois Institute of Technology. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. W-31-109-Eng-38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Unbiased electron density map of the MEK1 active site. (PDF 58 kb)
Supplementary Fig. 2
Mass distribution computed for the MEK, MgATP and PD318088 ternary complexes from analytical ultracentrifugation results. (PDF 110 kb)
Supplementary Fig. 3
Interference data from sedimentation equilibrium at 13 °C of the ternary complex of PD318088, MgATP with MEK1 (a, b) and MEK2 (c, d). (PDF 171 kb)
Supplementary Fig. 4
Multiple sequence alignment of the kinase domain of human MEK1 versus other members of the MKK family. (PDF 303 kb)
Supplementary Table 1
Apparent disassociation constants obtained from fitting the sedimentation equilibrium data for apo MEK1 and the ternary complex. (PDF 23 kb)
Supplementary Table 2
Apparent disassociation constants obtained from fitting the sedimentation equilibrium data for apo MEK2 and the ternary complex. (PDF 20 kb)
Supplementary Table 3
X–ray data collection and refinement statistics. (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Ohren, J., Chen, H., Pavlovsky, A. et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol 11, 1192–1197 (2004). https://doi.org/10.1038/nsmb859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb859
This article is cited by
-
Pancreatic cancer acquires resistance to MAPK pathway inhibition by clonal expansion and adaptive DNA hypermethylation
Clinical Epigenetics (2024)
-
Experimental strategies to improve drug-target identification in mass spectrometry-based thermal stability assays
Communications Chemistry (2023)
-
Erianin suppresses constitutive activation of MAPK signaling pathway by inhibition of CRAF and MEK1/2
Signal Transduction and Targeted Therapy (2023)
-
The cardiac glycoside ZINC253504760 induces parthanatos-type cell death and G2/M arrest via downregulation of MEK1/2 phosphorylation in leukemia cells
Cell Biology and Toxicology (2023)
-
MEK1/2 inhibition rescues neurodegeneration by TFEB-mediated activation of autophagic lysosomal function in a model of Alzheimer’s Disease
Molecular Psychiatry (2022)