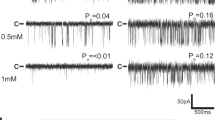
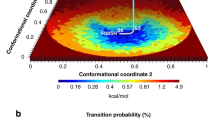
Abstract
RyR1 is an intracellular calcium channel with a central role in muscle contraction. We obtained a three-dimensional reconstruction of the RyR1 in the closed state at a nominal resolution of ∼10 Å using cryo-EM. The cytoplasmic assembly consists of a series of interconnected tubular structures that merge into four columns that extend into the transmembrane assembly. The transmembrane assembly, which has at least six transmembrane α-helices per monomer, has four tilted rods that can be fitted with the inner helices of a closed K+ channel atomic structure. The rods splay out at the lumenal side and converge into a dense ring at the cytoplasmic side. Another set of four rods emerges from this ring and shapes the inner part of the four columns. The resulting constricted axial structure provides direct continuity between cytoplasmic and transmembrane assemblies, and a possible mechanism for control of channel gating through conformational changes in the cytoplasmic assembly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Fill, M. & Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 82, 893–922 (2002).
Dulhunty, A.F. & Pouliquin, P. What we don't know about the structure of ryanodine receptor calcium release channels. Clin. Exp. Pharmacol. Physiol. 30, 713–723 (2003).
Wagenknecht, T. Samsó, M. Three-dimensional reconstruction of ryanodine receptors. Front. Biosci. 7, d1464–d1474 (2002).
Wolf, M., Eberhart, A., Glossmann, H., Striessnig, J. & Grigorieff, N. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J. Mol. Biol. 332, 171–182 (2003).
Paolini, C., Protasi, F. & Franzini-Armstrong, C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J. Mol. Biol. 342, 145–153 (2004).
Wagenknecht, T. et al. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J. Biol. Chem. 272, 32463–32471 (1997).
Samsó, M. & Wagenknecht, T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J. Biol. Chem. 277, 1349–1353 (2002).
Orlova, E.V. Serysheva, II, van Heel, M., Hamilton, S.L. & Chiu, W. Two structural configurations of the skeletal muscle calcium release channel. Nat. Struct. Biol. 3, 547–552 (1996).
Sharma, M.R., Jeyakumar, L.H., Fleischer, S. & Wagenknecht, T. Three-dimensional structure of ryanodine receptor isoform three in two conformational states as visualized by cryo-electron microscopy. J. Biol. Chem. 275, 9485–9491 (2000).
Radermacher, M. et al. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J. Cell Biol. 127, 411–423 (1994).
Serysheva, I.I. & Hamilton, S.L., Chiu, W. & Ludtke, S.J. Structure of Ca2+ release channel at 14 Å resolution. J. Mol. Biol. 345, 427–431 (2005).
Takeshima, H. et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339, 439–445 (1989).
Zorzato, F. et al. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 265, 2244–2256 (1990).
Grunwald, R. & Meissner, G. Lumenal sites and C terminus accessibility of the skeletal muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 270, 11338–11347 (1995).
Balshaw, D., Gao, L. & Meissner, G. Luminal loop of the ryanodine receptor: a pore-forming segment? Proc. Natl. Acad. Sci. USA 96, 3345–3347 (1999).
Zhao, M. et al. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 274, 25971–25974 (1999).
Welch, W., Rheault, S., West, D.J. & Williams, A.J. A model of the putative pore region of the cardiac ryanodine receptor channel. Biophys. J. 87, 2335–2351 (2004).
Du, G.G., Sandhu, B., Khanna, V.K., Guo, X.H. & MacLennan, D.H. Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1). Proc. Natl. Acad. Sci. USA 99, 16725–16730 (2002).
Wang, R. et al. The predicted TM10 transmembrane sequence of the cardiac Ca2+ release channel (ryanodine receptor) is crucial for channel activation and gating. J. Biol. Chem. 279, 3635–3642 (2004).
Williams, A.J., West, D.J. & Sitsapesan, R. Light at the end of the Ca2+-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels. Q. Rev. Biophys. 34, 61–104 (2001).
Doyle, D.A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Rosenthal, P.B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Malhotra, A. et al. Escherichia coli 70 S ribosome at 15 Å resolution by cryo-electron microscopy: localization of fMet-tRNAfMet and fitting of L1 protein. J. Mol. Biol. 280, 103–116 (1998).
Samsó, M., Trujillo, R., Gurrola, G.B., Valdivia, H.H. & Wagenknecht, T. Three-dimensional location of the imperatoxin A binding site on the ryanodine receptor. J. Cell Biol. 146, 493–499 (1999).
Jiang, W., Baker, M.L., Ludtke, S.J. & Chiu, W. Bridging the information gap: computational tools for intermediate resolution structure interpretation. J. Mol. Biol. 308, 1033–1044 (2001).
Fujiyoshi, Y. The structural study of membrane proteins by electron crystallography. Adv. Biophys. 35, 25–80 (1998).
Block, B.A., Imagawa, T., Campbell, K.P. & Franzini-Armstrong, C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107, 2587–2600 (1988).
Wagenknecht, T., Hsieh, C.E., Rath, B.K., Fleischer, S. & Marko, M. Electron tomo-graphy of frozen-hydrated isolated triad junctions. Biophys. J. 83, 2491–2501 (2002).
Meissner, G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 261, 6300–6306 (1986).
Unwin, N. Nicotinic acetylcholine receptor at 9 Å resolution. J. Mol. Biol. 229, 1101–1124 (1993).
Pessah, I.N. & Zimanyi, I. Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: evidence for a sequential mechanism in ryanodine action. Mol. Pharmacol. 39, 679–689 (1991).
Welch, W. et al. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry 36, 2939–2950 (1997).
Carroll, S., Skarmeta, J.G., Yu, X., Collins, K.D. & Inesi, G. Interdependence of ryanodine binding, oligomeric receptor interactions, and Ca2+ release regulation in junctional sarcoplasmic reticulum. Arch. Biochem. Biophys. 290, 239–247 (1991).
Fessenden, J.D. et al. Ryanodine receptor point mutant E4032A reveals an allosteric interaction with ryanodine. Proc. Natl. Acad. Sci. USA 98, 2865–2870 (2001).
Kobayashi, S., Yamamoto, T., Parness, J. & Ikemoto, N. Antibody probe study of Ca2+ channel regulation by interdomain interaction within the ryanodine receptor. Biochem. J. 380, 561–569 (2004).
Shtifman, A. et al. Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J. Gen. Physiol. 119, 15–32 (2002).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Wagenknecht, T. et al. Cryoelectron microscopy resolves FK506-binding protein sites on the skeletal muscle ryanodine receptor. Biophys. J. 70, 1709–1715 (1996).
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Grigorieff, N. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase (complex I) at 22 Å in ice. J. Mol. Biol. 277, 1033–1046 (1998).
Quillin, M.L. & Matthews, B.W. Accurate calculation of the density of proteins. Acta Crystallogr. D 56, 791–794 (2000).
Huang, C.C., Couch, G.S., Pettersen, E.F. & Ferrin, T.E. Chimera: an extensive molecular modeling application constructed using standard components. Pac. Symp. Biocomput. 1, 724 (1996).
Acknowledgements
We thank T. Walz for his generous sharing of the microscopy and image processing facilities at the Department of Cell Biology at Harvard Medical School, and for helpful discussions. We also acknowledge N. Grigorieff for allowing the use of CTFTILT and FREALIGN software, and the Resource for Visualization of Biological Complexity (US National Institutes of Health (NIH) RR01219) of Wadsworth Center for the use of the cryo-plunger apparatus. We thank Y. Cheng for his helpful discussions, advice with data analysis and technical support with the electron microscope, J. Frank for helpful discussions and X. Shen and J. Berkowitz for help with the RyR1 purification. This work was supported by RO1 AR43140 and PO1 AR17605 (to P.D.A.), AHA 0530147N (to M.S.), and AR40615 (to T.W.). The molecular EM facility at Harvard Medical School was established by a generous donation from the Giovanni Armenise Harvard Center for Structural Biology and is maintained by funds from NIH-GM62580 (to T. Walz).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Samsó, M., Wagenknecht, T. & Allen, P. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol 12, 539–544 (2005). https://doi.org/10.1038/nsmb938
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb938
This article is cited by
-
Regulatory mechanisms of ryanodine receptor/Ca2+ release channel revealed by recent advancements in structural studies
Journal of Muscle Research and Cell Motility (2021)
-
Resveratrol Directly Controls the Activity of Neuronal Ryanodine Receptors at the Single-Channel Level
Molecular Neurobiology (2020)
-
Voltage sensing mechanism in skeletal muscle excitation-contraction coupling: coming of age or midlife crisis?
Skeletal Muscle (2018)
-
The Central domain of RyR1 is the transducer for long-range allosteric gating of channel opening
Cell Research (2016)
-
Interaction of ions with the luminal sides of wild-type and mutated skeletal muscle ryanodine receptors
Journal of Molecular Modeling (2016)