Abstract
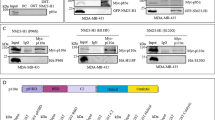
Phosphoinositide 3-kinase γ (PI3Kγ) consists of a catalytic subunit p110γ, which forms mutually exclusive dimers with one of the regulatory subunits called p101 and p84/p87PIKAP. Recently, PI3Kγ emerged as being a potential oncogene because overexpression of the catalytic subunit p110γ or the regulatory subunit p101 leads to oncogenic cellular transformation and malignancy. However, the contribution of the individual subunits to tumor growth and metastasis and the mechanisms involved are not understood. We therefore individually knocked down the PI3Kγ subunits (p84, p101 and p110γ) in MDA-MB-231 cells, which reduced in vitro migration of the cell lines. Knockdown of p110γ or p101 inhibited apoptosis, Akt phosphorylation and lung colonization in SCID mice. Similarly, the knockdown of p110γ and p101 in murine epithelial carcinoma 4T1.2 cells inhibited primary tumor growth and spontaneous metastasis, as well as lung colonization. In contrast, knockdown of p84 in MDA-MB-231 cells enhanced Akt phosphorylation and lung colonization. These findings are the first to implicate differential functions of the two PI3Kγ regulatory subunits in the process of oncogenesis, and indicate that loss of p101 is sufficient to reduce in vivo tumor growth and metastasis to the same extent as that of p110γ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Akekawatchai C, Holland JD, Kochetkova M, Wallace JC, McColl SR . (2005). Transactivation of CXCR4 by the insulin-like growth factor-1 receptor (IGF-1R) in human MDA-MB-231 breast cancer epithelial cells. J Biol Chem 280: 39701–39708.
al-Aoukaty A, Rolstad B, Maghazachi AA . (1999). Recruitment of pleckstrin and phosphoinositide 3-kinase gamma into the cell membranes, and their association with G beta gamma after activation of NK cells with chemokines. J Immunol 162: 3249–3255.
Aslakson CJ, Miller FR . (1992). Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 52: 1399–1405.
Bader AG, Kang S, Vogt PK . (2006). Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci USA 103: 1475–1479.
Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C et al. (2005). PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11: 933–935.
Benistant C, Chapuis H, Roche S . (2000). A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene 19: 5083–5090.
Bohnacker T, Marone R, Collmann E, Calvez R, Hirsch E, Wymann MP . (2009). PI3Kgamma adaptor subunits define coupling to degranulation and cell motility by distinct PtdIns(3,4,5)P3 pools in mast cells. Sci Signal 2: ra27.
Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann MP . (1998). Bifurcation of lipid and protein kinase signals of PI3Kgamma to the protein kinases PKB and MAPK. Science 282: 293–296.
Bretland AJ, Lawry J, Sharrard RM . (2001). A study of death by anoikis in cultured epithelial cells. Cell Prolif 34: 199–210.
Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K et al. (2003). Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol 160: 89–99.
Burgering BM, Coffer PJ . (1995). Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376: 599–602.
Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J et al. (2005). Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936–943.
Cantley LC . (2002). The phosphoinositide 3-kinase pathway. Science 296: 1655–1657.
Ching TT, Wang DS, Hsu AL, Lu PJ, Chen CS . (1999). Identification of multiple phosphoinositide-specific phospholipases D as new regulatory enzymes for phosphatidylinositol 3,4, 5-trisphosphate. J Biol Chem 274: 8611–8617.
Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O et al. (2007). Negative feedback regulation of Rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci USA 104: 14354–14359.
David M, Wannecq E, Descotes F, Jansen S, Deux B, Ribeiro J et al. (2010). Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS One 5: e9741.
Deane JA, Fruman DA . (2004). Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol 22: 563–598.
Denley A, Gymnopoulos M, Kang S, Mitchell C, Vogt PK . (2009). Requirement of phosphatidylinositol(3,4,5)trisphosphate in phosphatidylinositol 3-kinase-induced oncogenic transformation. Mol Cancer Res 7: 1132–1138.
Denley A, Kang S, Karst U, Vogt PK . (2008). Oncogenic signaling of class I PI3K isoforms. Oncogene 27: 2561–2574.
Dhand R, Hara K, Hiles I, Bax B, Gout I, Panayotou G et al. (1994). PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J 13: 511–521.
Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD et al. (2005). Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res 3: 1–13.
Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H et al. (2010). Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clin Cancer Res 16: 4928–4937.
El Haibi CP, Sharma PK, Singh R, Johnson PR, Suttles J, Singh S et al. (2010). PI3Kp110-, Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells. Mol Cancer 9: 85.
Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S et al. (1998). The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem 273: 23169–23175.
Gillooly DJ, Simonsen A, Stenmark H . (2001). Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J 355: 249–258.
Goldberg GS, Jin Z, Ichikawa H, Naito A, Ohki M, El-Deiry WS et al. (2001). Global effects of anchorage on gene expression during mammary carcinoma cell growth reveal role of tumor necrosis factor-related apoptosis-inducing ligand in anoikis. Cancer Res 61: 1334–1337.
Hawkins PT, Anderson KE, Davidson K, Stephens LR . (2006). Signalling through class I PI3Ks in mammalian cells. Biochem Soc Trans 34: 647–662.
Holland JD, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl SR . (2006). Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res 66: 4117–4124.
Johnson C, Marriott SJ, Levy LS . (2007). Overexpression of p101 activates PI3Kgamma signaling in T cells and contributes to cell survival. Oncogene 26: 7049–7057.
Kang S, Denley A, Vanhaesebroeck B, Vogt PK . (2006). Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103: 1289–1294.
Kim JH, Adelstein RS . (2011). LPA(1) -induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. J Cell Physiol 226: 2881–2893.
Knobbe CB, Reifenberger G . (2003). Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol 13: 507–518.
Kochetkova M, Kumar S, McColl SR . (2009). Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ 16: 664–673.
Krugmann S, Hawkins PT, Pryer N, Braselmann S . (1999). Characterizing the interactions between the two subunits of the p101/p110gamma phosphoinositide 3-kinase and their role in the activation of this enzyme by G beta gamma subunits. J Biol Chem 274: 17152–17158.
Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P et al. (1999). A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis 17: 163–170.
Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nurnberg B . (1998). Gbetagamma stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273: 7024–7029.
Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R . (1997). Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science 275: 394–397.
Maier U, Babich A, Nurnberg B . (1999). Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms beta and gamma. J Biol Chem 274: 29311–29317.
Metjian A, Roll RL, Ma AD, Abrams CS . (1999). Agonists cause nuclear translocation of phosphatidylinositol 3-kinase gamma. A Gbetagamma-dependent pathway that requires the p110gamma amino terminus. J Biol Chem 274: 27943–27947.
Mizoguchi M, Nutt CL, Mohapatra G, Louis DN . (2004). Genetic alterations of phosphoinositide 3-kinase subunit genes in human glioblastomas. Brain Pathol 14: 372–377.
Nicholson KM, Anderson NG . (2002). The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14: 381–395.
Okkenhaug K, Vanhaesebroeck B . (2003). PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 3: 317–330.
Orme MH, Alrubaie S, Bradley GL, Walker CD, Leevers SJ . (2006). Input from Ras is required for maximal PI(3)K signalling in Drosophila. Nat Cell Biol 8: 1298–1302. Epub 15 October 2006.
Osaki M, Oshimura M, Ito H . (2004). PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 9: 667–676.
Pons S, Asano T, Glasheen E, Miralpeix M, Zhang Y, Fisher TL et al. (1995). The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol Cell Biol 15: 4453–4465.
Procko E, McColl SR . (2005). Leukocytes on the move with phosphoinositide 3-kinase and its downstream effectors. Bioessays 27: 153–163.
Riccardi C, Nicoletti I . (2006). Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1: 1458–1461.
Rodrigues DH, Vilela MC, Barcelos LS, Pinho V, Teixeira MM, Teixeira AL . (2010). Absence of PI3Kgamma leads to increased leukocyte apoptosis and diminished severity of experimental autoimmune encephalomyelitis. J Neuroimmunol 222: 90–94.
Savagner P . (2010). The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol 21 (Suppl 7): vii89–vii92.
Shi M, Zheng Y, Garcia A, Xu L, Foster DA . (2007). Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer Lett 258: 268–275.
Simonsen A, Wurmser AE, Emr SD, Stenmark H . (2001). The role of phosphoinositides in membrane transport. Curr Opin Cell Biol 13: 485–492.
Sotsios Y, Whittaker GC, Westwick J, Ward SG . (1999). The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol 163: 5954–5963.
Stephens L-R, Equinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J et al. (1997). The G-beta-gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89: 105–114.
Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens L . (2005). p84, a new Gbetagamma-activated regulatory subunit of the type IB phosphoinositide 3-kinase p110gamma. Curr Biol 15: 566–570.
Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K et al. (2006). Gbetagammas and the Ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol 8: 1303–1309.
Tang MK, Zhou HY, Yam JW, Wong AS . (2010). c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia 12: 128–138.
Tao K, Fang M, Alroy J, Sahagian GG . (2008). Imagable 4T1 model for the study of late stage breast cancer. BMC cancer 8: 228.
Valentijn AJ, Zouq N, Gilmore AP . (2004). Anoikis. Biochem Soc Trans 32: 421–425.
Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC et al. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602.
Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP et al. (2000). Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6: 909–919.
Walker EH, Perisic O, Ried C, Stephens L, Williams RL . (1999). Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 402: 313–320.
Wang LH . (2004). Molecular signaling regulating anchorage-independent growth of cancer cells. Mt Sinai J Med 71: 361–367.
Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR . (2010). Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer 9: 250.
Wymann MP, Zvelebil M, Laffargue M . (2003). Phosphoinositide 3-kinase signalling—which way to target? Trends Pharmacol Sci 24: 366–376.
Yamada M, Banno Y, Takuwa Y, Koda M, Hara A, Nozawa Y . (2004). Overexpression of phospholipase D prevents actinomycin D-induced apoptosis through potentiation of phosphoinositide 3-kinase signalling pathways in Chinese-hamster ovary cells. Biochem J 378: 649–656.
Yu G, Shen FS, Sturch S, Aquino A, Glazer RI, Felsted RL . (1995). Regulation of HIV-1 gag protein subcellular targeting by protein kinase C. J Biol Chem 270: 4792–4796.
Zhao L, Vogt PK . (2008). Class I PI3K in oncogenic cellular transformation. Oncogene 27: 5486–5496.
Zhao M, Mueller BM, DiScipio RG, Schraufstatter IU . (2008). Akt plays an important role in breast cancer cell chemotaxis to CXCL12. Breast Cancer Res Treat 110: 211–222.
Zhong M, Shen Y, Zheng Y, Joseph T, Jackson D, Foster DA . (2003). Phospholipase D prevents apoptosis in v-Src-transformed rat fibroblasts and MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun 302: 615–619.
Acknowledgements
We thank Mark Bunting for assistance with animal experiments, John Brazzatti for assistance with statistical analysis and Francisco Olmo for assistance with apoptosis experiments. The work was supported by grants from the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Brazzatti, J., Klingler-Hoffmann, M., Haylock-Jacobs, S. et al. Differential roles for the p101 and p84 regulatory subunits of PI3Kγ in tumor growth and metastasis. Oncogene 31, 2350–2361 (2012). https://doi.org/10.1038/onc.2011.414
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.414
Keywords
This article is cited by
-
Varied functions of immune checkpoints during cancer metastasis
Cancer Immunology, Immunotherapy (2021)
-
LncRNA CANT1 suppresses retinoblastoma progression by repellinghistone methyltransferase in PI3Kγ promoter
Cell Death & Disease (2020)
-
Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration
Leukemia (2018)
-
p84 forms a negative regulatory complex with p110γ to control PI3Kγ signalling during cell migration
Immunology & Cell Biology (2015)
-
The relationship between mTOR signalling pathway and recombinant antibody productivity in CHO cell lines
BMC Biotechnology (2014)