Abstract
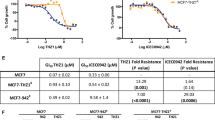
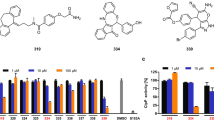
Protein kinase CK2 is an ubiquitous and constitutively active kinase, which phosphorylates many cellular proteins and is implicated in the regulation of cell survival, proliferation and transformation. We investigated its possible involvement in the multidrug resistance phenotype (MDR) by analysing its level in two variants of CEM cells, namely S-CEM and R-CEM, normally sensitive or resistant to chemical apoptosis, respectively. We found that, while the CK2 regulatory subunit β was equally expressed in the two cell variants, CK2α catalytic subunit was higher in R-CEM and this was accompanied by a higher phosphorylation of endogenous protein substrates. Pharmacological downregulation of CK2 activity by a panel of specific inhibitors, or knockdown of CK2α expression by RNA interference, were able to induce cell death in R-CEM. CK2 inhibitors could promote an increased uptake of chemotherapeutic drugs inside the cells and sensitize them to drug-induced apoptosis in a co-operative manner. CK2 blockade was also effective in inducing cell death of a different MDR line (U2OS). We therefore conclude that inhibition of CK2 can be considered as a promising tool to revert the MDR phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- 2a:
-
2-amino-4,5,6,7-tetrabromo-1H-benzimidazole
- CK2:
-
casein kinase 2
- IQA:
-
5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl]acetic acid
- MDR:
-
multidrug resistance
- MNA:
-
1,8-dihydroxy-4-nitro-anthracene-9,10-dione
- MNX:
-
1,8-dihydroxy-4-nitro-xanthen-9-one
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltriazolium bromide
- P-gp:
-
P-glycoprotein
- R-CEM:
-
multidrug resistant CEM
- S-CEM:
-
wild type CEM
- siRNA:
-
short interference RNA
- TBB:
-
4,5,6,7-tetrabromobenzotriazole
- TBI:
-
4,5,6,7-tetrabromobenzimidazole
- Vbl:
-
vinblastine
References
Ahmad KA, Harris NH, Johnson AD, Lindvall HC, Wang G, Ahmed K . (2007). Protein kinase CK2 modulates apoptosis induced by resveratrol and epigallocatechin-3-gallate in prostate cancer cells. Mol Cancer Ther 6: 1006–1012.
Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K . (2005). Targeting CK2 for cancer therapy. Anticancer Drugs 16: 1037–1043.
Ahmed K, Gerber DA, Cochet C . (2002). Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12: 226–230.
Allende CC, Allende JE . (1998). Promiscuous subunit interactions: a possible mechanism for the regulation of protein kinase CK2. J Cell Biochem Suppl 30-31: 129–136.
Borst P . (1997). Multidrug resistant proteins. Semin Cancer Biol 8: 131–134.
Cenni V, Maraldi NM, Ruggeri A, Secchiero P, Del Coco R, De Pol A et al. (2004). Sensitization of multidrug resistant human ostesarcoma cells to Apo2 Ligand/TRAIL-induced apoptosis by inhibition of the Akt/PKB kinase. Int J Oncol 25: 1599–1608.
De Moliner E, Moro S, Sarno S, Zagotto G, Zanotti G, Pinna LA et al. (2003). Inhibition of protein kinase CK2 by anthraquinone-related compounds. A structural insight. J Biol Chem 278: 1831–1836.
Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F et al. (2005). Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ 12: 668–677.
Dupuis ML, Tombesi M, Sabatini M, Cianfriglia M . (2003). Differential effect of HIV-1 protease inhibitors on P-glycoprotein function in multidrug-resistant variants of the human CD4+ T lymphoblastoid CEM cell line. Chemotherapy 49: 8–16.
Filhol O, Martiel JL, Cochet C . (2004). Protein kinase CK2: a new view of an old molecular complex. EMBO Rep 5: 351–355.
Giovannetti E, Mey V, Danesi R, Mosca I, Del Tacca M . (2004). Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res 10: 2936–2943.
Glavy JS, Horwitz SB, Orr GA . (1997). Identification of the in vivo phosphorylation sites for acidic-directed kinases in murine mdr1b P-glycoprotein. J Biol Chem 272: 5909–5914.
Guerra B, Issinger OG . (1999). Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20: 391–408.
Guo C, Yu S, Davis AT, Wang H, Green JE, Ahmed K . (2001). A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem 276: 5992–5999.
Kelliher MA, Seldin DC, Leder P . (1996). Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J 15: 5160–5166.
Klumpp S, Maurer A, Zhu Y, Aichele D, Pinna LA, Krieglstein J . (2004). Protein kinase CK2 phosphorylates BAD at threonine-117. Neurochem Int. 45: 747–752.
Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC . (1998). p53 deficiency and misexpression of protein kinase CK2alpha collaborate in the development of thymic lymphomas in mice. Oncogene 16: 2965–2974.
Ling V . (1997). Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol 40: S3–S8.
Litchfield DW . (2003). Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369: 1–15.
Matsumoto Y, Takano H, Kunishio K, Nagao S, Fojo T . (2001). Expression of drug resistance genes in VP-16 and mAMSA-selected human carcinoma cells. Jpn J Cancer Res 92: 778–784.
Meggio F, Pinna LA . (2003). One-thousand-and-one substrates of protein kinase CK2? FASEB J 17: 349–368.
Meggio F, Pagano MA, Moro S, Zagotto G, Ruzzene M, Sarno S et al. (2004). Inhibition of protein kinase CK2 by condensed polyphenolic derivatives. An in vitro and in vivo study. Biochemistry 43: 12931–12936.
Orlandini M, Semplici F, Ferruzzi R, Meggio F, Pinna LA, Oliviero S . (1998). Protein kinase CK2alpha′ is induced by serum as a delayed early gene and cooperates with Ha-ras in fibroblast transformation. J Biol Chem 273: 21291–21297.
Pagano MA, Andrzejewska M, Ruzzene M, Sarno S, Cesaro L, Bain J et al. (2004). Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J Med Chem 47: 6239–6247.
Pinna LA . (1990). Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim Biophys Acta 1054: 267–284.
Pinna LA . (2002). Protein kinase CK2: a challenge to canons. J Cell Sci 115: 3873–3878.
Ruzzene M, Penzo D, Pinna LA . (2002). Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J 364: 41–47.
Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA . (2006). Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett 580: 3948–3952.
Sarno S, de Moliner E, Ruzzene M, Pagano MA, Battistutta R, Bain J et al. (2003). Biochemical and three-dimensional-structural study of the specific inhibition of protein kinase CK2 by [5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl]acetic acid (IQA). Biochem J 374: 639–646.
Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A et al. (2001). Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’). FEBS Lett 496: 44–48.
Sarno S, Ruzzene M, Frascella P, Pagano MA, Meggio F, Zambon A et al. (2005). Development and exploitation of CK2 inhibitors. Mol Cell Biochem 274: 69–76.
Sarno S, Salvi M, Battistutta R, Zanotti G, Pinna LA . (2005a). Features and potentials of ATP-site directed CK2 inhibitors. Biochim Biophys Acta 1754: 263–270.
Sawicka M, Kalinowska M, Skierski J, Lewandowski W . (2004). A review of selected anti-tumour therapeutic agents and reasons for multidrug resistance occurrence. J Pharm Pharmacol 56: 1067–1081.
Seeber S, Issinger OG, Holm T, Kristensen LP, Guerra B . (2005). Validation of protein kinase CK2 as oncological target. Apoptosis 10: 875–885.
Seldin DC, Leder P . (1995). Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science 267: 894–897.
Szyszka R, Grankowski N, Felczak K, Shugar D . (1995). Halogenated benzimidazoles and benzotriazoles as selective inhibitors of protein kinases CK I and CK II from Saccharomyces cerevisiae and other sources. Biochem Biophys Res Commun 208: 418–424.
Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF . (2006). Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA 103: 15079–15084.
Acknowledgements
We thank Dr S Sarno (Padova) for providing recombinant CK2, and Dr S Klumpp (Munster) for providing anti Tp117-BAD. The work was supported by grants from University of Padova (Progetto Ateneo 2005) to MR and from AIRC and EC (PRO-KINASERESEARCH 503467) to LAP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Maira, G., Brustolon, F., Bertacchini, J. et al. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene 26, 6915–6926 (2007). https://doi.org/10.1038/sj.onc.1210495
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210495
Keywords
This article is cited by
-
Protein kinase CK2: a potential therapeutic target for diverse human diseases
Signal Transduction and Targeted Therapy (2021)
-
Targeting CK2 in cancer: a valuable strategy or a waste of time?
Cell Death Discovery (2021)
-
Role of protein kinase CK2 in antitumor drug resistance
Journal of Experimental & Clinical Cancer Research (2019)
-
Therapeutic targeting of CK2 in acute and chronic leukemias
Leukemia (2018)
-
Protein kinase CK2 in breast cancer: the CK2β regulatory subunit takes center stage in epithelial plasticity
Cellular and Molecular Life Sciences (2015)