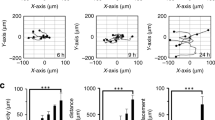
Abstract
Endoglin is a transforming growth factor β (TGFβ) superfamily auxiliary receptor. We had previously shown that it suppressed prostate cancer (PCa) cell motility, and that its expression was lost during PCa progression. The mechanism by which endoglin inhibits PCa cell motility is unknown. Here we demonstrate that endoglin abrogates TGFβ-mediated cell motility, but does not alter cell surface binding of TGFβ. By measuring Smad-specific phosphorylation and Smad-responsive promoter activity, endoglin was shown to constitutively activate Smad1, with little-to-no effect upon Smad3. Knockdown of Smad1 increased motility and abrogated endoglin's effects. As type I activin receptor-like kinases (ALKs) are necessary for Smad activation, we went on to show that knockdown of ALK2, but not TGFβRI (ALK5), abrogated endoglin-mediated decreases in cell motility and constitutively active ALK2 was sufficient to restore a low-motility phenotype in endoglin deficient cells. These findings provide the first evidence that endoglin decreases PCa cell motility through activation of the ALK2-Smad1 pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Attisano L, Carcamo J, Ventura F, Weis FM, Massague J, Wrana JL . (1993). Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75: 671–680.
Barbara NP, Wrana JL, Letarte M . (1999). Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem 274: 584–594.
Bergan R, Hakim F, Schwartz GN, Kyle E, Cepada R, Szabo JM et al. (1996a). Electroporation of synthetic oligodeoxynucleotides: a novel technique for ex vivo bone marrow purging. Blood 88: 731–741.
Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L . (1996b). Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis 14: 389–398.
Bissell MJ, Radisky D . (2001). Putting tumours in context. Nat Rev Cancer 1: 46–54.
Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C . (2005). Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol 204: 574–584.
Carroll PR, Lee KL, Fuks ZY, Kantoff PW . (2001). CANCER: Principals and Practices of Oncology.In: Devita Vt, Hellman S, Rosenberg Sa (eds). Lippincott-Raven: New York, pp 1418–1479.
Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J et al. (1992). Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 267: 19027–19030.
de Caestecker MP, Hemmati P, Larisch-Bloch S, Ajmera R, Roberts AB, Lechleider RJ . (1997). Characterization of functional domains within Smad4/DPC4. J Biol Chem 272: 13690–13696.
Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM . (1998). Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17: 3091–3100.
Desgrosellier JS, Mundell NA, McDonnell MA, Moses HL, Barnett JV . (2005). Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol 280: 201–210.
Ding Y, Xu L, Chen S, Jovanovic BD, Helenowski IB, Kelly DL et al. (2006). Characterization of a method for profiling gene expression in cells recovered from intact human prostate tissue using RNA linear amplification. Prostate Cancer Prostatic Dis 9: 379–391.
Ebner R, Chen RH, Lawler S, Zioncheck T, Derynck R . (1993a). Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science 262: 900–902.
Ebner R, Chen RH, Shum L, Lawler S, Zioncheck TF, Lee A et al. (1993b). Cloning of a type I TGF-beta receptor and its effect on TGF-beta binding to the type II receptor. Science 260: 1344–1348.
Gougos A, Letarte M . (1990). Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem 265: 8361–8364.
Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C et al. (2003). Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 12: 817–828.
Hayes SA, Huang X, Kambhampati S, Platanias LC, Bergan RC . (2003). p38 MAP kinase modulates Smad-dependent changes in human prostate cell adhesion. Oncogene 22: 4841–4850.
Huang X, Chen S, Xu L, Liu YQ, Deb DK, Platanias LC et al. (2005). Genistein inhibits p38 MAP kinase activation, MMP-2, and cell invasion in human prostate epithelial cells. Cancer Res 65: 3470–3478.
Jovanovic BD, Huang S, Liu Y, Naguib KN, Bergan RC . (2001). A simple analysis of gene expression and variability in gene arrays based on repeated observations. Am J Pharmacogenomics 1: 145–152.
Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR . (1984). Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res 44: 3522–3529.
Lastres P, Letamendia A, Zhang H, Rius C, Almendro N, Raab U et al. (1996). Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol 133: 1109–1121.
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M et al. (2004). Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 23: 4018–4028.
Letamendia A, Lastres P, Botella LM, Raab U, Langa C, Velasco B et al. (1998). Role of endoglin in cellular responses to transforming growth factor-beta. A comparative study with betaglycan. J Biol Chem 273: 33011–33019.
Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC . (2002). Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene 21: 8272–8281.
Liu YQ, Kyle E, Patel S, Housseau F, Hakim F, Lieberman R et al. (2001). Prostate cancer chemoprevention agents exhibit selective activity against early stage prostate cancer cells. Prostate Cancer Prostatic Dis 4: 81–91.
Massague J . (1998). TGF-beta signal transduction. Annu Rev Biochem 67: 753–791.
Miettinen PJ, Ebner R, Lopez AR, Derynck R . (1994). TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 127: 2021–2036.
Monteiro RM, de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL . (2004). Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci 117: 4653–4663.
Pece-Barbara N, Vera S, Kathirkamathamby K, Liebner S, Di Guglielmo GM, Dejana E et al. (2005). Endoglin null endothelial cells proliferate faster and are more responsive to transforming growth factor beta1 with higher affinity receptors and an activated Alk1 pathway. J Biol Chem 280: 27800–27808.
Poste G, Fidler IJ . (1980). The pathogenesis of cancer metastasis. Nature 283: 139–146.
Shi Y, Massague J . (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700.
ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H et al. (1993). Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 8: 2879–2887.
Ulloa L, Doody J, Massague J . (1999). Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397: 710–713.
Ward SM, Desgrosellier JS, Zhuang X, Barnett JV, Galper JB . (2002). Transforming growth factor beta (TGFbeta) signaling via differential activation of activin receptor-like kinases 2 and 5 during cardiac development. Role in regulating parasympathetic responsiveness. J Biol Chem 277: 50183–50189.
Wieser R, Wrana JL, Massague J . (1995). GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J 14: 2199–2208.
Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M et al. (1992). TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014.
Xu L, Chen S, Bergan RC . (2006). MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 25: 2987–2998.
Acknowledgements
We express our gratitude to Dr Shan Chen for her many helpful suggestions throughout the manuscript's preparation. This work was funded by the following grants to Raymond C Bergan: a merit review award from the Veterans Administration and a Specialized Program of Research Excellence (SPORE) grant CA90386, from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Clarissa S Craft was funded by a training grant, T32CA09560, from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene Web site (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Craft, C., Romero, D., Vary, C. et al. Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway. Oncogene 26, 7240–7250 (2007). https://doi.org/10.1038/sj.onc.1210533
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210533
Keywords
This article is cited by
-
Recurrences in stage II rectal carcinoma after curative resection alone: from the viewpoint of angiogenesis
World Journal of Surgical Oncology (2016)
-
Identification of Endoglin as an epigenetically regulated tumour-suppressor gene in lung cancer
British Journal of Cancer (2015)
-
PRH/HHex inhibits the migration of breast and prostate epithelial cells through direct transcriptional regulation of Endoglin
Oncogene (2014)
-
Thyroid hormone suppresses cell proliferation through endoglin-mediated promotion of p21 stability
Oncogene (2013)
-
Endoglin expression in breast tumor cells suppresses invasion and metastasis and correlates with improved clinical outcome
Oncogene (2011)