-
PDF
- Split View
-
Views
-
Cite
Cite
Mabrouka Doghman, Tatiana Karpova, Giovanna Assis Rodrigues, Malika Arhatte, Juliana De Moura, Luciane R. Cavalli, Virginie Virolle, Pascal Barbry, Gerard P. Zambetti, Bonald C. Figueiredo, Leslie L. Heckert, Enzo Lalli, Increased Steroidogenic Factor-1 Dosage Triggers Adrenocortical Cell Proliferation and Cancer, Molecular Endocrinology, Volume 21, Issue 12, 1 December 2007, Pages 2968–2987, https://doi.org/10.1210/me.2007-0120
Close - Share Icon Share
Abstract
Steroidogenic factor-1 (SF-1/Ad4BP; NR5A1), a nuclear receptor transcription factor, has a pivotal role in adrenal and gonadal development in humans and mice. A frequent feature of childhood adrenocortical tumors is SF-1 amplification and overexpression. Here we show that an increased SF-1 dosage can by itself augment human adrenocortical cell proliferation through concerted actions on the cell cycle and apoptosis. This effect is dependent on an intact SF-1 transcriptional activity. Gene expression profiling showed that an increased SF-1 dosage regulates transcripts involved in steroid metabolism, the cell cycle, apoptosis, and cell adhesion to the extracellular matrix. Consistent with these results, increased SF-1 levels selectively modulate the steroid secretion profile of adrenocortical cells, reducing cortisol and aldosterone production and maintaining dehydroepiandrosterone sulfate secretion. As a model to understand the mechanisms of transcriptional regulation by increased SF-1 dosage, we studied FATE1, coding for a cancer-testis antigen implicated in the control of cell proliferation. Increased SF-1 levels increase its binding to a consensus site in FATE1 promoter and stimulate its activity through modulation of the recruitment of specific cofactors. On the other hand, sphingosine, which can compete with phospholipids for binding to SF-1, had no effect on the SF-1 dosage-dependent increase of adrenocortical cell proliferation and expression of the FATE1 promoter. In mice, increased Sf-1 dosage produces adrenocortical hyperplasia and formation of tumors expressing gonadal markers (Amh, Gata-4), which originate from the subcapsular region of the adrenal cortex. Gene expression profiling revealed that genes involved in cell adhesion and the immune response and transcription factor signal transducer and activator of transcription-3 (Stat3) are differentially expressed in Sf-1 transgenic mouse adrenals compared with wild-type adrenals. Our studies reveal a critical role for SF-1 dosage in adrenocortical tumorigenesis and constitute a rationale for the development of drugs targeting SF-1 transcriptional activity for adrenocortical tumor therapy.
ADRENOCORTICAL TUMORS (ACT) in children are found isolated or associated with other types of cancers in the context of genetically determined syndromes (1). ACT incidence in children is highest during the first 3 yr of life. These tumors are 10–15 times more frequent in southern Brazil than in the rest of the world (2). In that region, it is almost invariably found associated with a specific germline TP53 (tumor protein p53) mutation (R337H) (3). This mutation is predicted to impair p53 function by inducing a pH-dependent destabilization of protein tetramers (4).
ACTs most commonly present in children with a virilization syndrome due to production of androgenic steroids by the tumor, which can be associated with signs of excess glucocorticoid production. Therapeutic results are still unsatisfactory, with an overall survival rate at 5 yr of only about 55%. Favorable prognostic factors are stage I at diagnosis, tumor weight of 200 g or less, age younger than 4 yr, and presence of virilization alone (5). Childhood ACT are thought to be derived from the fetal adrenal because of their age distribution, their pattern of hormone secretion, and their molecular phenotype (5, 6). During the first months of neonatal life, the human fetal adrenal cortex undergoes a massive remodeling process. Definitive zone cells lying in the outer cortex proliferate and differentiate into the glomerulosa, fasciculata, and reticularis zones concomitantly with regression of the inner androgenic steroid-secreting fetal zone (7). It has been shown that an apoptotic process is responsible for fetal zone regression after birth (8), and childhood ACTs have been hypothesized to be caused by defective apoptosis (6).
ACT may also occur in ferrets and in certain mouse strains after gonadectomy (see Ref. 9 for review). In mice, gonadectomy-induced ACT are found either in certain inbred (e.g. C3H and DBA/2J) or in specific laboratory-engineered strains [inhibin α-null mice and inhibin α promoter-SV40 T antigen transgenic (TR) mice] (10, 11). A common feature of all types of gonadectomy-induced ACT occurring in rodents is the gonadal phenotype of the neoplastic cells, which express gonadal markers (e.g. AMH, LHR, and Gata-4) and produce androgens and estradiol (10, 12). Increased gonadotropin production in gonadectomized animals has been shown to play a fundamental role in triggering adrenocortical tumorigenesis in susceptible strains (10, 13–16), and expression of a single-chain human chorionic gonadotropin was shown to trigger adrenocortical neoplastic changes in a susceptible strain even in the absence of gonadectomy (14).
Adrenal glands and gonads are derived from the same embryological primordium. Several transcriptional regulators play an important role for the genesis of both organs (reviewed in Ref. 17). In particular, nuclear receptor steroidogenic factor-1 (SF-1/Ad4BP; NR5A1) has a pivotal role (18, 19). In mice, adrenal size is directly proportional to Sf-1 gene dosage (20–24). A direct relationship between Sf-1 copy number and adrenal proliferative potential is also shown by the impaired compensatory adrenal growth in Sf-1 +/− animals (25). In humans, SF-1 mutations have been described in patients with various degrees of adrenal and gonadal dysgenesis (for a review see Ref. 26).
We have shown previously that the SF-1 gene is amplified and overexpressed in childhood ACT and hypothesized that it may play an important role in the genesis and progression of those tumors (27, 28). Here we show that increased SF-1 dosage augments proliferation and diminishes apoptosis of human adrenocortical cells and produces ACT in mice. These results indicate that SF-1 dosage may be critical for adrenal tumorigenesis and suggest that modulation of SF-1 activity may represent an important therapeutic target in childhood ACT.
RESULTS
SF-1 Overexpression Increases Proliferation in Human Adrenocortical Cells
A significant increase in SF-1 gene copy number (27) and protein (28) has been observed in childhood ACT bearing the germline TP53 R337H mutation. Increased SF-1 levels do not correlate with clinical severity of the disease or histological grading. Unfortunately, cell lines derived from childhood ACT and bearing the TP53 R337H mutation are lacking. Consequently, to dissect the cellular and molecular consequences of increased levels of SF-1 in human adrenal cells, we overexpressed wild-type (WT) and mutant [S203A, A433W, and L451A/L452A (AF-2mut)] SF-1 proteins in an inducible fashion in the H295R adrenal cell line. S203A and A433W SF-1 mutants have an impaired capacity to be activated via the MAPK pathway (29) or to bind phospholipids (30), respectively, whereas the transactivation activity of AF-2mut SF-1 is severely diminished (31). H295R cells are differentiated to produce a panel of steroid hormones, have a fetal adrenal phenotype (32), and express SF-1 endogenously (33). In addition, TP53 gene sequencing revealed that H295R cells are homozygous for the R72P polymorphism and heterozygous for the F338L mutation in TP53 (data not shown). This latter mutation affects the residue adjacent to R337 in the TP53 C-terminal tetramerization domain and is sensitive to inactivating mutations in the yeast assay (34).
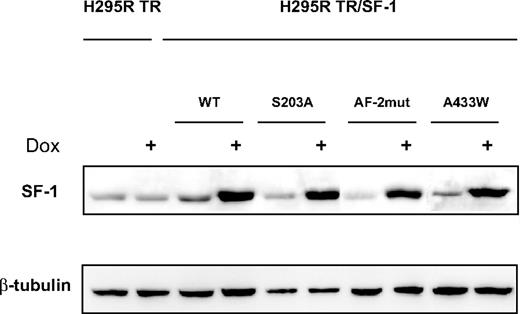
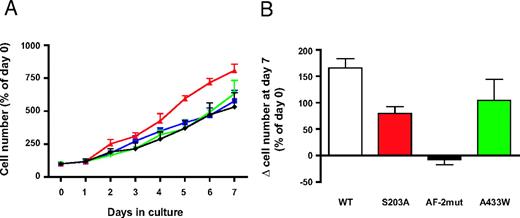
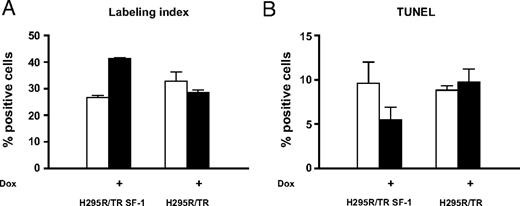
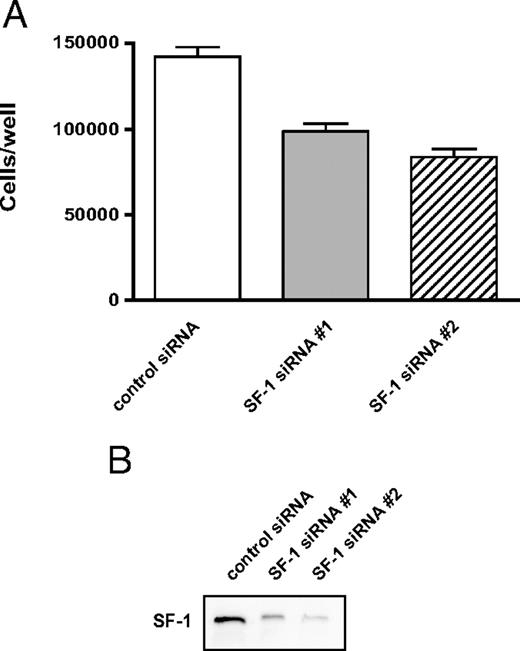
WT and mutant SF-1 protein levels are increased to similar levels in H295R TR/SF-1 cells after doxycycline (a stable tetracycline analog) treatment, whereas, as expected, no SF-1 induction is triggered by doxycycline in the H295R TR parental clone (Fig. 1). We monitored the effect of SF-1 dosage on proliferation capacities of H295R TR and H295R TR/SF-1 WT cells. Doxycycline treatment significantly increases proliferation of H295R TR/SF-1 WT cells but has no effect on H295R TR cells. (Fig. 2A). To rule out clonal effects, these results were confirmed using two independently isolated H295R TR/SF-1 WT cell clones (data not shown). AF-2-dependent transactivation activity of SF-1 is necessary to activate proliferation. The S203A and A433W mutants were still partially able to activate proliferation in a doxycycline-dependent manner when overexpressed in H295R cells (Fig. 2B). We then asked whether the increased number of H295R TR/SF-1 WT cells determined by SF-1 overexpression is due to increased proliferation, decreased numbers of apoptotic cells, or both. Doxycycline treatment of H295R TR/SF-1 WT cells significantly increases the number of bromodeoxyuridine (BrdU)-positive cells (Fig. 3A) and decreases the number of apoptotic cells (Fig. 3B), whereas it has no effect on the parental H295R TR cells. To assess how modulation of endogenous SF-1 levels influences H295R cell proliferation, we compared the growth rate of cells where SF-1 expression was knocked down by either one of two different specific small interfering RNA (siRNA) and of cells treated with a control siRNA. Both SF-1-specific siRNA significantly reduce SF-1 protein expression and H295R cell proliferation, as compared with cells treated with control siRNA (Fig. 4).
An Inducible Cellular System for SF-1 Overexpression in Human Adrenocortical Cells SF-1 expression is activated by doxycycline (Dox) treatment (1 μg/ml for 72 h) in H295R TR/SF-1 WT, S203A, AF-2mut, and A433W cells but not in parental H295R TR cells. β-Tubulin expression is shown as a control.
SF-1 Overexpression Increases Adrenocortical Cell Proliferation A, H295R TR and H295R TR/SF-1 WT cells were cultured in duplicate wells in the absence or in the presence of doxycycline (1 μg/ml) in the culture medium for a 7-d period and counted every day. Data are expressed as percentage of the number of cells on the day when cultures were started (d 0). Green inverted triangles, H295R TR; black diamonds, H295R TR plus doxycycline; blue squares, H295R TR/SF-1 WT; red triangles, H295R TR/SF-1 WT plus doxycycline. sem is indicated. The difference in cell numbers between H295R TR/SF-1 cells cultured in the absence and in the presence of doxycycline is statistically significant (P = 0.0156, Wilcoxon signed rank test; n = 3), whereas the difference between H295R TR cell numbers in the absence and in the presence of doxycycline is not. B, Effect of SF-1 mutants on cell proliferation. H295R TR clones overexpressing SF-1S203A, SF-1AF-2mut, or SF-1A433W were cultured in duplicate in the presence or in the absence of doxycycline (1 μg/ml). At d 7, their number, expressed as the difference (percentage of d 0) between treatments with or without doxycycline, was compared with H295R TR/SF-1 WT cells grown in the same conditions. A significant difference was found only for SF-1AF-2mut cells (P = 0.0015, Kruskal-Wallis test; n = 4).
An Increase in SF-1 Dosage Augments S-Phase and Reduces Apoptosis in Human Adrenocortical Cells Percentage of BrdU-positive (labeling index) (A) and terminal dUTP nick end-labeling (TUNEL)-positive H295R TR/SF-1 WT cells (B) cultured for 72 h in the presence (black histograms) or in the absence (white histograms) of doxycycline (1 μg/ml). Data are derived from three experiments where more than 1000 cells were counted in total for each condition. The differences between cells cultured in the presence and in the absence of doxycycline are significant for both labeling index and TUNEL staining (P < 0.0001 and P = 0.0031, respectively, Fisher’s exact test).
Knockdown of Endogenous SF-1 in H295R Cells Reduces Their Proliferation A, H295R cells were transfected in triplicate wells with two different SF-1-specific siRNA (gray and hatched histograms, respectively) or with a control, GC-content-matched siRNA (white histogram), using a double transfection protocol (transfection on d 1 and 4). Cell numbers were counted on d 6. sem is indicated. The difference between cell numbers on d 6 is statistically significant (P = 0.0390, Kruskal-Wallis test; n = 3). B, Immunoblot showing efficient knockdown of SF-1 on d 6 in cells transfected with either SF-1-specific siRNA.
Increased SF-1 Dosage Selectively Modulates Steroidogenesis in H295R Cells
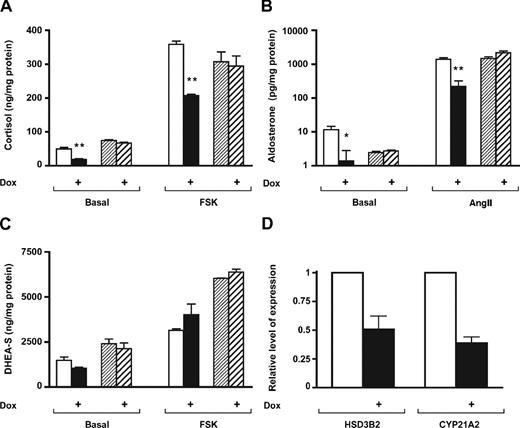
SF-1 is considered as a master regulator of steroid hormone production by activating the expression of steroidogenic enzymes (18). On the other hand, in some settings, steroid production by adrenocortical cells is inversely proportional to their proliferative activity (35). To assess whether steroidogenic capacities of H295R TR/SF-1 cells can be modulated by an increase in SF-1 dosage, we monitored their production of cortisol, aldosterone, and dehydroepiandrosterone sulfate (DHEA-S) in basal and stimulated conditions in the presence and absence of doxycycline. We observed a significant decrease in basal and stimulated cortisol and aldosterone secretion when SF-1 was overexpressed, whereas there was no significant change in DHEA-S levels. Conversely, doxycycline treatment induced no significant differences in basal or stimulated cortisol, aldosterone, and DHEA-S production in parental H295R TR cells (Fig. 5, A–C). Consistent with the pattern of steroid production by doxycycline-treated H295R TR/SF-1 cells, levels of HSD3B2 and CYP21A2 transcripts were down-regulated in conditions of increased SF-1 expression (Fig. 5D).
Steroid Secretion Is Selectively Modulated by Increased SF-1 Dosage in Human Adrenocortical Cells Cortisol (A), aldosterone (B), and DHEA-S (C) were assayed in the culture medium of H295R/TR and H295R TR/SF-1 cells in basal conditions or stimulated with 10 μg/ml forskolin (FSK) (for cortisol and DHEA-S assays) or 10 nm angiotensin II (AngII) (for aldosterone assays) for 48 h in the absence (white histograms, H295R TR/SF-1 cells; finely hatched bars, H295R/TR cells) or in the presence (black histograms, H295R TR/SF-1 cells; coarsely hatched bars, H295R/TR cells) of doxycycline (Dox; 1 μg/ml). Steroids were assayed in triplicate by specific RIA (aldosterone and DHEA-S) or chemiluminescent assays (cortisol). sem is indicated. Note that the y-axis in B is in log scale. Significant differences existed between cortisol levels (P = 0.0041 for basal and P = 0.0001 for forskolin-stimulated levels, t test; n = 3) and aldosterone levels (P = 0.0320 for basal and P = 0.0031 for angiotensin II-stimulated levels, t test; n = 3) in the absence and in the presence of doxycycline. *, P < 0.05; **, P < 0.01. No significant difference for all measured steroids existed in basal vs. doxycycline-treated parental H295R/TR cells. D, Relative expression of HSD3B2 and CYP21A2 mRNAs was assayed by qRT-PCR in H295R TR/SF-1 cells cultured for 72 h in the absence (white histograms) or in the presence (black histograms) of doxycycline (1 μg/ml).
Transcripts Regulated by Increased SF-1 Dosage in H295R Cells
To get insight into the molecular mechanisms underlying the effect of SF-1 overexpression upon adrenocortical cell proliferation, we performed gene expression profiling in two different H295R TR/SF-1 WT cell clones (untreated or treated with doxycycline) and identified transcripts whose levels are significantly modulated by increased SF-1 dosage at 3 d after the start of doxycycline treatment (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). To validate microarray results, expression of a subset of transcripts was measured by quantitative RT-PCR (qRT-PCR). The results obtained using this technique were highly correlated with the microarray results (supplemental Fig. 1). Analysis of gene expression regulated by SF-1 dosage in H295R cells by the DAVID (http://niaid.abcc.ncifcrf.gov/) software (Table 1) showed a significant enrichment of transcripts belonging to the following Gene Ontology categories.
Gene Ontology Categories and Pathways Significantly Regulated in H295R Cells by Increased SF-1 Dosage
| Category . | Term . | P Value . | Genes . |
|---|---|---|---|
| GOTERM_BP_ALL | Steroid metabolism | 0.001 | CYP21A2, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| SP_PIR_KEYWORDS | Signal | 0.001 | SERPINA5, DCBLD2, COL15A1, APOA1, CDH22, FXYD6, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008161:collagen helix repeat | 0.002 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Extracellular matrix | 0.004 | COL15A1, CHAD, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Lipid metabolism | 0.004 | ACSL6, SORL1, APOA1, SULT2A1 |
| SP_PIR_KEYWORDS | Glycoprotein | 0.004 | ENPP2, SERPINA5, DCBLD2, ITGA1, COL15A1, ATP1B1, CDH22 TAS2R49, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008160:collagen triple helix repeat | 0.006 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Collagen | 0.007 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Steroid metabolism | 0.008 | SORL1, APOA1, SULT2A1 |
| KEGG_PATHWAY | HSA04510:focal adhesion | 0.009 | ITGA1, CHAD, CCND3, COL11A1, VAV2, PDGFD |
| INTERPRO_NAME | IPR013320:ConA-like lectin/glucanase | 0.010 | COL15A1, COL18A1, COL11A1, NPTX2 |
| GOTERM_BP_ALL | Phosphate transport | 0.010 | COL15A1, COL18A1, COL11A1, EMID1 |
| INTERPRO_NAME | IPR010515:collagenase NC10 and endostatin collagen | 0.012 | COL15A1, COL18A1 |
| GOTERM_CC_ALL | Collagen | 0.014 | COL15A1, COL18A1, COL11A1 |
| GOTERM_BP_ALL | Regulation of progression through cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| GOTERM_BP_ALL | Regulation of cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| SP_PIR_KEYWORDS | Membrane | 0.016 | ENPP2, FATE1, DCBLD2, CYP21A2, ACSL6, ITGA1, AKAP7, CYP3A7, ATP1B1, CDH22, TAS2R49, FXYD6, TM7SF3, TNFRSF19, ABCA3, AMIGO2, PTPRZ1, RAB31, SORL1, SLC31A2, AXL |
| GOTERM_BP_ALL | Cellular lipid metabolism | 0.019 | CYP21A2, ACSL6, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| GOTERM_CC_ALL | Microsome | 0.024 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_BP_ALL | Induction of programmed cell death | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Induction of apoptosis | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_CC_ALL | Vesicular fraction | 0.025 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_CC_ALL | Extracellular region | 0.033 | SERPINA5, NOV, BMP6, COL15A1, FGF13, CHAD, COL18A1, APOA1, AKR1B1, COL11A1, EMID1, FXYD6 |
| GOTERM_MF_ALL | Growth factor activity | 0.033 | NOV, BMP6, FGF13, PDGFD |
| GOTERM_BP_ALL | Positive regulation of Apoptosis | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Positive regulation of programmed cell death | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| SP_PIR_KEYWORDS | Cell adhesion | 0.035 | ITGA1, COL15A1, AMIGO2, COL18A1, CDH22 |
| GOTERM_BP_ALL | Inorganic anion transport | 0.035 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Transcription factor | 0.038 | GATA3, BHLHB3, ETV1, NR0B1 |
| GOTERM_BP_ALL | Steroid biosynthesis | 0.043 | CYP21A2, HSD11B2, NR0B1 |
| GOTERM_BP_ALL | Transport | 0.048 | NXN, CYP21A2, COL15A1, AKAP7, SLC16A10, APOA1, CYP3A7, ATP1B1, FXYD6, SYTL2, ABCA3, RAB31, COL18A1, SORL1, COL11A1, EMID1, SLC31A2, TUBB, RIMS3 |
| Category . | Term . | P Value . | Genes . |
|---|---|---|---|
| GOTERM_BP_ALL | Steroid metabolism | 0.001 | CYP21A2, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| SP_PIR_KEYWORDS | Signal | 0.001 | SERPINA5, DCBLD2, COL15A1, APOA1, CDH22, FXYD6, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008161:collagen helix repeat | 0.002 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Extracellular matrix | 0.004 | COL15A1, CHAD, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Lipid metabolism | 0.004 | ACSL6, SORL1, APOA1, SULT2A1 |
| SP_PIR_KEYWORDS | Glycoprotein | 0.004 | ENPP2, SERPINA5, DCBLD2, ITGA1, COL15A1, ATP1B1, CDH22 TAS2R49, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008160:collagen triple helix repeat | 0.006 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Collagen | 0.007 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Steroid metabolism | 0.008 | SORL1, APOA1, SULT2A1 |
| KEGG_PATHWAY | HSA04510:focal adhesion | 0.009 | ITGA1, CHAD, CCND3, COL11A1, VAV2, PDGFD |
| INTERPRO_NAME | IPR013320:ConA-like lectin/glucanase | 0.010 | COL15A1, COL18A1, COL11A1, NPTX2 |
| GOTERM_BP_ALL | Phosphate transport | 0.010 | COL15A1, COL18A1, COL11A1, EMID1 |
| INTERPRO_NAME | IPR010515:collagenase NC10 and endostatin collagen | 0.012 | COL15A1, COL18A1 |
| GOTERM_CC_ALL | Collagen | 0.014 | COL15A1, COL18A1, COL11A1 |
| GOTERM_BP_ALL | Regulation of progression through cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| GOTERM_BP_ALL | Regulation of cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| SP_PIR_KEYWORDS | Membrane | 0.016 | ENPP2, FATE1, DCBLD2, CYP21A2, ACSL6, ITGA1, AKAP7, CYP3A7, ATP1B1, CDH22, TAS2R49, FXYD6, TM7SF3, TNFRSF19, ABCA3, AMIGO2, PTPRZ1, RAB31, SORL1, SLC31A2, AXL |
| GOTERM_BP_ALL | Cellular lipid metabolism | 0.019 | CYP21A2, ACSL6, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| GOTERM_CC_ALL | Microsome | 0.024 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_BP_ALL | Induction of programmed cell death | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Induction of apoptosis | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_CC_ALL | Vesicular fraction | 0.025 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_CC_ALL | Extracellular region | 0.033 | SERPINA5, NOV, BMP6, COL15A1, FGF13, CHAD, COL18A1, APOA1, AKR1B1, COL11A1, EMID1, FXYD6 |
| GOTERM_MF_ALL | Growth factor activity | 0.033 | NOV, BMP6, FGF13, PDGFD |
| GOTERM_BP_ALL | Positive regulation of Apoptosis | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Positive regulation of programmed cell death | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| SP_PIR_KEYWORDS | Cell adhesion | 0.035 | ITGA1, COL15A1, AMIGO2, COL18A1, CDH22 |
| GOTERM_BP_ALL | Inorganic anion transport | 0.035 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Transcription factor | 0.038 | GATA3, BHLHB3, ETV1, NR0B1 |
| GOTERM_BP_ALL | Steroid biosynthesis | 0.043 | CYP21A2, HSD11B2, NR0B1 |
| GOTERM_BP_ALL | Transport | 0.048 | NXN, CYP21A2, COL15A1, AKAP7, SLC16A10, APOA1, CYP3A7, ATP1B1, FXYD6, SYTL2, ABCA3, RAB31, COL18A1, SORL1, COL11A1, EMID1, SLC31A2, TUBB, RIMS3 |
P value is the EASE score, a modified Fisher’s exact test to measure significance of gene enrichment in annotation terms (http://niaid.abcc.ncifcrf.gov/helps/functional_annotation.html#E3).
Gene Ontology Categories and Pathways Significantly Regulated in H295R Cells by Increased SF-1 Dosage
| Category . | Term . | P Value . | Genes . |
|---|---|---|---|
| GOTERM_BP_ALL | Steroid metabolism | 0.001 | CYP21A2, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| SP_PIR_KEYWORDS | Signal | 0.001 | SERPINA5, DCBLD2, COL15A1, APOA1, CDH22, FXYD6, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008161:collagen helix repeat | 0.002 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Extracellular matrix | 0.004 | COL15A1, CHAD, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Lipid metabolism | 0.004 | ACSL6, SORL1, APOA1, SULT2A1 |
| SP_PIR_KEYWORDS | Glycoprotein | 0.004 | ENPP2, SERPINA5, DCBLD2, ITGA1, COL15A1, ATP1B1, CDH22 TAS2R49, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008160:collagen triple helix repeat | 0.006 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Collagen | 0.007 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Steroid metabolism | 0.008 | SORL1, APOA1, SULT2A1 |
| KEGG_PATHWAY | HSA04510:focal adhesion | 0.009 | ITGA1, CHAD, CCND3, COL11A1, VAV2, PDGFD |
| INTERPRO_NAME | IPR013320:ConA-like lectin/glucanase | 0.010 | COL15A1, COL18A1, COL11A1, NPTX2 |
| GOTERM_BP_ALL | Phosphate transport | 0.010 | COL15A1, COL18A1, COL11A1, EMID1 |
| INTERPRO_NAME | IPR010515:collagenase NC10 and endostatin collagen | 0.012 | COL15A1, COL18A1 |
| GOTERM_CC_ALL | Collagen | 0.014 | COL15A1, COL18A1, COL11A1 |
| GOTERM_BP_ALL | Regulation of progression through cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| GOTERM_BP_ALL | Regulation of cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| SP_PIR_KEYWORDS | Membrane | 0.016 | ENPP2, FATE1, DCBLD2, CYP21A2, ACSL6, ITGA1, AKAP7, CYP3A7, ATP1B1, CDH22, TAS2R49, FXYD6, TM7SF3, TNFRSF19, ABCA3, AMIGO2, PTPRZ1, RAB31, SORL1, SLC31A2, AXL |
| GOTERM_BP_ALL | Cellular lipid metabolism | 0.019 | CYP21A2, ACSL6, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| GOTERM_CC_ALL | Microsome | 0.024 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_BP_ALL | Induction of programmed cell death | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Induction of apoptosis | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_CC_ALL | Vesicular fraction | 0.025 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_CC_ALL | Extracellular region | 0.033 | SERPINA5, NOV, BMP6, COL15A1, FGF13, CHAD, COL18A1, APOA1, AKR1B1, COL11A1, EMID1, FXYD6 |
| GOTERM_MF_ALL | Growth factor activity | 0.033 | NOV, BMP6, FGF13, PDGFD |
| GOTERM_BP_ALL | Positive regulation of Apoptosis | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Positive regulation of programmed cell death | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| SP_PIR_KEYWORDS | Cell adhesion | 0.035 | ITGA1, COL15A1, AMIGO2, COL18A1, CDH22 |
| GOTERM_BP_ALL | Inorganic anion transport | 0.035 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Transcription factor | 0.038 | GATA3, BHLHB3, ETV1, NR0B1 |
| GOTERM_BP_ALL | Steroid biosynthesis | 0.043 | CYP21A2, HSD11B2, NR0B1 |
| GOTERM_BP_ALL | Transport | 0.048 | NXN, CYP21A2, COL15A1, AKAP7, SLC16A10, APOA1, CYP3A7, ATP1B1, FXYD6, SYTL2, ABCA3, RAB31, COL18A1, SORL1, COL11A1, EMID1, SLC31A2, TUBB, RIMS3 |
| Category . | Term . | P Value . | Genes . |
|---|---|---|---|
| GOTERM_BP_ALL | Steroid metabolism | 0.001 | CYP21A2, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| SP_PIR_KEYWORDS | Signal | 0.001 | SERPINA5, DCBLD2, COL15A1, APOA1, CDH22, FXYD6, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008161:collagen helix repeat | 0.002 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Extracellular matrix | 0.004 | COL15A1, CHAD, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Lipid metabolism | 0.004 | ACSL6, SORL1, APOA1, SULT2A1 |
| SP_PIR_KEYWORDS | Glycoprotein | 0.004 | ENPP2, SERPINA5, DCBLD2, ITGA1, COL15A1, ATP1B1, CDH22 TAS2R49, NPTX2, TM7SF3, TNFRSF19, NOV, AMIGO2, BMP6, PTPRZ1, CHAD, COL18A1, SORL1, COL11A1, EMID1, AXL |
| INTERPRO_NAME | IPR008160:collagen triple helix repeat | 0.006 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Collagen | 0.007 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Steroid metabolism | 0.008 | SORL1, APOA1, SULT2A1 |
| KEGG_PATHWAY | HSA04510:focal adhesion | 0.009 | ITGA1, CHAD, CCND3, COL11A1, VAV2, PDGFD |
| INTERPRO_NAME | IPR013320:ConA-like lectin/glucanase | 0.010 | COL15A1, COL18A1, COL11A1, NPTX2 |
| GOTERM_BP_ALL | Phosphate transport | 0.010 | COL15A1, COL18A1, COL11A1, EMID1 |
| INTERPRO_NAME | IPR010515:collagenase NC10 and endostatin collagen | 0.012 | COL15A1, COL18A1 |
| GOTERM_CC_ALL | Collagen | 0.014 | COL15A1, COL18A1, COL11A1 |
| GOTERM_BP_ALL | Regulation of progression through cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| GOTERM_BP_ALL | Regulation of cell cycle | 0.015 | PPP1R13B, CDKN2D, DUSP6, CCND3, MLLT7, SNF1LK, AXL |
| SP_PIR_KEYWORDS | Membrane | 0.016 | ENPP2, FATE1, DCBLD2, CYP21A2, ACSL6, ITGA1, AKAP7, CYP3A7, ATP1B1, CDH22, TAS2R49, FXYD6, TM7SF3, TNFRSF19, ABCA3, AMIGO2, PTPRZ1, RAB31, SORL1, SLC31A2, AXL |
| GOTERM_BP_ALL | Cellular lipid metabolism | 0.019 | CYP21A2, ACSL6, SORL1, APOA1, SULT2A1, HSD11B2, NR0B1 |
| GOTERM_CC_ALL | Microsome | 0.024 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_BP_ALL | Induction of programmed cell death | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Induction of apoptosis | 0.025 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_CC_ALL | Vesicular fraction | 0.025 | CYP21A2, ACSL6, CYP3A7, HSD11B2 |
| GOTERM_CC_ALL | Extracellular region | 0.033 | SERPINA5, NOV, BMP6, COL15A1, FGF13, CHAD, COL18A1, APOA1, AKR1B1, COL11A1, EMID1, FXYD6 |
| GOTERM_MF_ALL | Growth factor activity | 0.033 | NOV, BMP6, FGF13, PDGFD |
| GOTERM_BP_ALL | Positive regulation of Apoptosis | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| GOTERM_BP_ALL | Positive regulation of programmed cell death | 0.034 | TNFRSF19, PPP1R13B, TUBB, CIDEA |
| SP_PIR_KEYWORDS | Cell adhesion | 0.035 | ITGA1, COL15A1, AMIGO2, COL18A1, CDH22 |
| GOTERM_BP_ALL | Inorganic anion transport | 0.035 | COL15A1, COL18A1, COL11A1, EMID1 |
| SP_PIR_KEYWORDS | Transcription factor | 0.038 | GATA3, BHLHB3, ETV1, NR0B1 |
| GOTERM_BP_ALL | Steroid biosynthesis | 0.043 | CYP21A2, HSD11B2, NR0B1 |
| GOTERM_BP_ALL | Transport | 0.048 | NXN, CYP21A2, COL15A1, AKAP7, SLC16A10, APOA1, CYP3A7, ATP1B1, FXYD6, SYTL2, ABCA3, RAB31, COL18A1, SORL1, COL11A1, EMID1, SLC31A2, TUBB, RIMS3 |
P value is the EASE score, a modified Fisher’s exact test to measure significance of gene enrichment in annotation terms (http://niaid.abcc.ncifcrf.gov/helps/functional_annotation.html#E3).
Lipid and Steroid Metabolism.
Although it is not surprising to find represented this category of genes because of the known role of SF-1 in regulating steroidogenic gene expression, it is remarkable that an increased SF-1 dosage appears to selectively down-regulate genes involved in adult-type adrenal steroidogenesis and up-regulate genes involved in fetal steroidogenesis (see below).
Apoptosis and Cell Cycle Regulation.
A significant number of transcripts with a role in apoptosis and/or cell cycle progression is regulated by increased SF-1 dosage in H295R cells. This is consistent with the effect of increased SF-1 dosage on S-phase progression and percentage of apoptotic cells (Fig. 3). However, functional annotation of genes involved in regulation of apoptosis and cell cycle progression is most likely still preliminary. This is shown by the example of nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a secreted multimodular protein that is powerfully down-regulated in H295R cells by increased SF-1 dosage. We have recently shown that NOV/CCN3 is a selective proapoptotic factor for adrenocortical cells whose expression is also strikingly reduced in childhood ACT, independently from their clinical outcome (36, 37).
Cell Adhesion and Extracellular Matrix.
One unexpected result from our gene expression profiling experiments is that SF-1 dosage regulates the expression of a set of genes encoding molecules involved in cell adhesion and interaction with the extracellular matrix. Cell adhesion pathways are known to be important modulators of cell growth in development and cancer (see Ref. 38 for review), and their modulation has probably an important role in the activation of proliferation triggered by an increased SF-1 dosage. Remarkably, as revealed by pathway analysis (http://www.genome.ad.jp/kegg/pathway.html), SF-1 regulates the expression of several genes whose products lie in the same focal adhesion pathway (adhesion molecules ITGA1 and CHAD, extracellular matrix component COL11A1, growth factor PDGFD, protooncogene VAV2 implicated in Rac/Rho activation, and cyclin CCND3).
Transcription Factors.
It is remarkable that an increased SF-1 dosage up-regulates, among other transcription factors, the expression of DAX-1 (NR0B1). This factor is a direct target for SF-1 and regulates negatively its activity (39). DAX-1 overexpression triggered by increased SF-1 levels may represent a mechanism to limit the consequences of excessive SF-1 stimulation.
Mechanisms of Transcriptional Regulation by Increased SF-1 Dosage
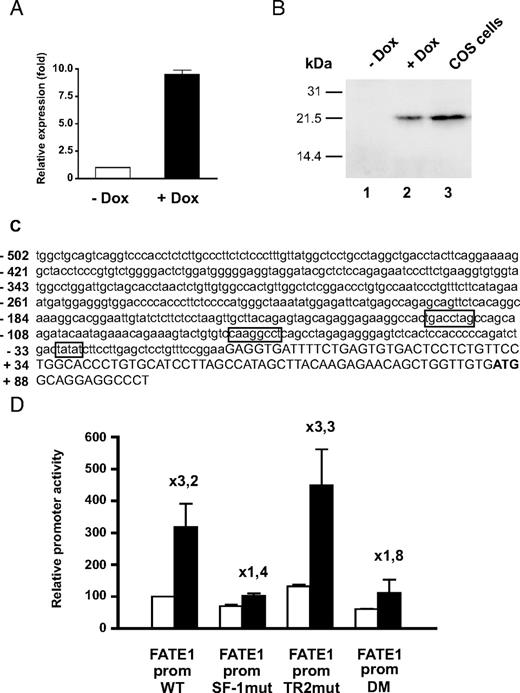
As a model to understand the molecular mechanisms involved in gene expression regulation by SF-1 dosage, we studied the FATE1 gene, whose levels are strongly up-regulated in H295R/TR SF-1 cells after doxycycline stimulation. The X-linked FATE1 gene encodes a transcript originally identified as highly expressed in testis (40) and subsequently shown to be a cancer-testis antigen expressed in hepatocellular carcinomas. Its expression is inversely correlated with their differentiation state (41, 42). Transfection of fetal and adult testis expressed-1 (FATE1) increases and anti-FATE1 antibodies decreases cell proliferation in hepatocarcinoma cells (42). In H295R/TR SF-1 cells, FATE1 expression is induced at both the transcript (Fig. 6A) and protein levels (Fig. 6B) by SF-1 overexpression. The FATE1 promoter harbors a consensus SF-1 binding site at positions −78/−71 relative to the transcription start site; another half-site motif for nuclear receptors (TR2), which is another potential SF-1 binding site, was identified by Matinspector software (Genomatix) analysis at positions −122/−115 from the transcription start site (Fig. 6C). We cloned the FATE1 promoter in front of the luciferase gene and measured its activity in H295R/TR SF-1 cells cultured in basal conditions or after doxycycline stimulation to increase SF-1 expression. WT FATE1 promoter activity is stimulated by increased SF-1 dosage in H295R TR/SF-1 cells (Fig. 6D). A mutant promoter fragment where the consensus SF-1 site was mutagenized (SF-1mut) has a slightly reduced activity in H295R TR/SF-1 cells in the absence of doxycycline, compared with the WT promoter, whereas an increased SF-1 dosage only marginally increases its activity. Conversely, FATE1 promoter harboring a mutated nuclear receptor half-site (TR2mut) responds to an increase in SF-1 dosage, similar to the WT promoter. Furthermore, combined mutations of both the SF-1 consensus and the nuclear receptor half-sites (DM) have no further effect on FATE1 promoter inducibility by increased SF-1 dosage than the single mutation of the SF-1 consensus binding site. These data show that the integrity of the −78/−71 consensus SF-1 binding site is the major determinant of increased FATE1 promoter activity in H295R TR/SF-1 cells in the presence of an increased SF-1 dosage.
FATE1 Is Up-Regulated by Increased SF-1 Dosage in H295R TR/SF-1 Cells through a Consensus SF-1 Binding Site Present in Its Promoter A, Relative FATE1 mRNA expression in H295R TR/SF-1 cells cultured in basal conditions or with doxycycline (Dox; 1 μg/ml) added in the culture medium. Cells cultured in triplicate wells were assayed after 3 d of culture. FATE1 mRNA levels are expressed relative to the quantity present in cells cultured in basal conditions. B, FATE1 protein expression in H295R TR/SF-1 cells cultured in basal conditions (lane 1), in the presence of doxycycline (Dox; 1 μg/ml) for 3 d (lane 2) and in COS cells transfected with a FATE1 expression vector. Heavy membrane fractions were purified and assayed for FATE1 expression by immunoblot. C, Nucleotide sequence of the FATE1 promoter. The TATA sequence, consensus SF-1 binding site, and nuclear receptor half-site are boxed. The sequence after the transcription start site is in uppercase. The ATG encoding Met1 in the protein is in bold. D, Activity of WT and mutant FATE1 promoter-luciferase constructs in H295R TR/SF-1 cells cultured in basal conditions (white bars) or with doxycycline (1 μg/ml; black bars) added in the culture medium. DM, Double mutant harboring both SF-1 consensus and nuclear receptor half-site mutations; prom, promoter; SF-1mut, SF-1 consensus binding site mutant; TR2mut, nuclear receptor half-site mutant. Fold induction of construct activity after increase of SF-1 dosage is indicated.
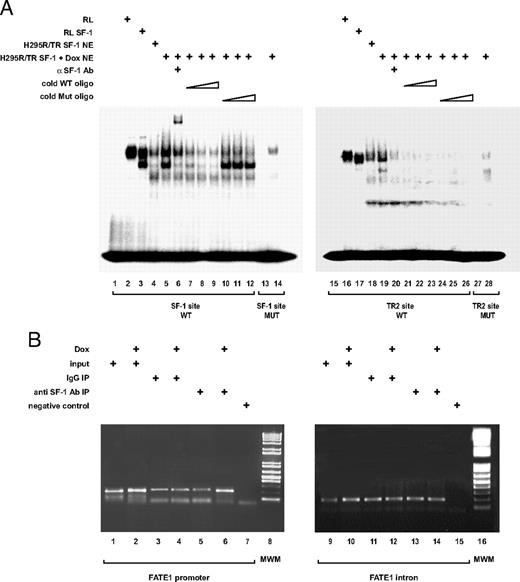
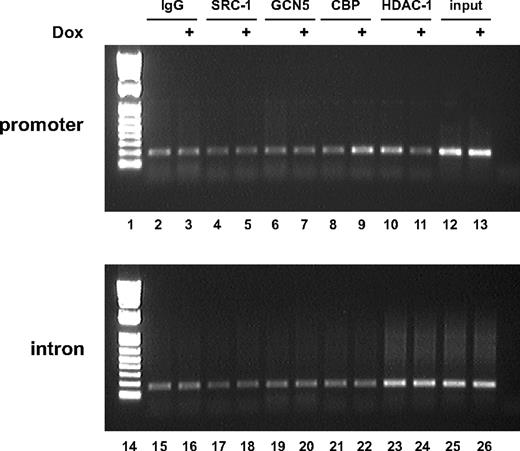
We then analyzed whether an increased SF-1 dosage translates into an increased recruitment of this transcription factor to the FATE1 promoter. As shown in Fig. 7A, in an EMSA, nuclear extracts prepared from doxycycline-stimulated H295R TR/SF-1 cells (lane 5) bind more strongly to a DNA oligonucleotide harboring the FATE1 promoter consensus SF-1 binding site than nuclear extracts prepared from H295R TR/SF-1 cells cultured in basal conditions (lane 4). The complex formed between H295R TR/SF-1 nuclear extracts and the SF-1 binding site oligonucleotide comigrates with the complex formed between the same oligonucleotide and SF-1 produced by in vitro translation (lane 3). Furthermore, the complex formed between H295R TR/SF-1 nuclear extracts and the SF-1 binding site oligonucleotide contains endogenous SF-1, as shown by supershift with an anti-SF-1 antibody (lane 6). Complex formation is competed by increasing doses of cold WT SF-1 binding site oligonucleotide (lanes 7–9) but not by the same oligonucleotide harboring mutations that impair SF-1 binding (lanes 10–12). In addition, no specific complex is formed between H295R TR/SF-1 nuclear extracts prepared from doxycycline-stimulated cells and a mutated SF-1 binding site oligonucleotide (lane 14). On the other hand, H295R TR/SF-1 nuclear extracts bind only poorly to a DNA oligonucleotide harboring the nuclear receptor half-site (TR2) present in the FATE1 promoter (lanes 18 and 19). This binding can be readily competed by both WT and mutated cold oligonucleotide (lanes 21–26). Similar to the results with the SF-1 oligonucleotide, H295R TR/SF-1 nuclear extracts do not bind specifically to a mutated TR2 oligonucleotide (lane 28). SF-1 binding to the endogenous FATE1 promoter or to a fragment of its second intron, taken as a control, was also assayed by chromatin immunoprecipitation (ChIP) (Fig. 7B). SF-1 association with its promoter is increased by doxycycline treatment of H295R TR/SF-1 cells compared with cells cultured in basal conditions (lanes 5 and 6), whereas no enrichment of SF-1 binding to the intronic fragment can be detected after doxycycline treatment of cells (lanes 13 and 14). SF-1 elicits target gene transcription through dynamic interaction with a large array of transcriptional intermediary factors or cofactors (19, 43–45). By ChIP assay, we could show that an increased SF-1 dosage in H295R TR/SF-1 cells could increase cAMP response element-binding protein-binding protein (CBP) and decrease histone deacetylase 1 (HDAC1) recruitment to the FATE1 promoter, whereas binding of the steroid receptor coactivator-1 (SRC-1) and general control nonderepressed-5 (GCN5) coactivators to the promoter did not change compared with cells cultured in basal conditions (Fig. 8, lanes 2–13). In all cases, no modulation of cofactor binding to FATE1 second intron by doxycycline treatment could be detected (lanes 15–26).
Increase of SF-1 Dosage in H295R TR/SF-1 Cells Increases Recruitment of SF-1 to the Consensus SF-1 Binding Site in the FATE1 Promoter A, Gel mobility shift showing binding of SF-1 to the WT consensus SF-1 binding site (lanes 1–12), mutated SF-1 binding site (lanes 13 and 14), WT nuclear receptor half-site (lanes 15–26), and mutated half-site (lanes 27 and 28). Lanes 1, 13, 15, and 27, free probes; lanes 2 and 16, unprogrammed rabbit reticulocyte lysate (RL); lanes 3 and 17, in vitro translated SF-1; lanes 4 and 18, nuclear extract (NE; 6 μg) from H295R TR/SF-1 cells cultured in basal conditions; lanes 5–12 and 19–26, nuclear extract (6 μg) from H295R TR/SF-1 cells cultured in the presence of doxycycline: supershift using an anti-SF-1 antibody (lanes 6 and 20), competition by increasing doses of WT consensus SF-1 binding site (lanes 7–9) and nuclear receptor half-site (lanes 21–23) cold oligonucleotides (oligo), and competition by increasing doses of mutated SF-1 binding site (lanes 10–12) and nuclear receptor half-site (lanes 24–26) cold oligonucleotides. B, ChIP assay showing recruitment of SF-1 to the FATE1 promoter (lanes 1–7) and to the second intron of the gene (lanes 9–15) in H295R TR/SF-1 cells cultured in basal conditions (lanes 1, 3, 5, 9, 11, and 13) or treated with doxycycline (Dox; 1 μg/ml) to induce SF-1 expression (lanes 2, 4, 6, 10, 12, and 14). Input (lanes 1, 2, 9, and 10), 1:10 input chromatin; lanes 7 and 15, negative controls (no chromatin). ChIP were performed using rabbit IgG as a control (lanes 3, 4, 11, and 12) or an anti SF-1 antibody (Ab) raised in rabbit (lanes 5, 6, 13, and 14). Lanes 8 and 16, Molecular weight markers.
Different Cofactors Are Differentially Recruited to the FATE1 Promoter in Conditions of Increased SF-1 Dosage ChIP assay showing cofactors recruitment to the FATE1 promoter (lanes 2–13) and to the second intron of the gene (lanes 15–26) in H295R TR/SF-1 cells cultured in basal conditions (lanes 2, 4, 6, 8, 10, 12, 15, 17, 19, 21, 23, and 25) or treated with doxycycline (Dox; 1 μg/ml) to induce SF-1 expression (lanes 3, 5, 7, 9, 11, 13, 16, 18, 20, 22, 24, and 26). Input (lanes 12, 13, 25, and 26), 1:10 input chromatin. ChIP were performed using rabbit IgG as a control (lanes 2, 3, 15, and 16) or anti-SRC-1 (lanes 4, 5, 17, and 18), anti-GCN5 (lanes 6, 7, 19, and 20), anti-CBP (lanes 8, 9, 21, and 22), and anti-HDAC1 (lanes 10, 11, 23, and 24) antibodies. Lanes 1 and 14, Molecular weight markers.
All together, these results show that an increased SF-1 dosage in H295R TR/SF-1 cells increases its binding to a consensus binding site in the FATE1 promoter and stimulates promoter activity through modulation of the recruitment of specific cofactors.
Cell Proliferation and Gene Regulation Controlled by an Increased SF-1 Dosage Are Insensitive to Sphingosine
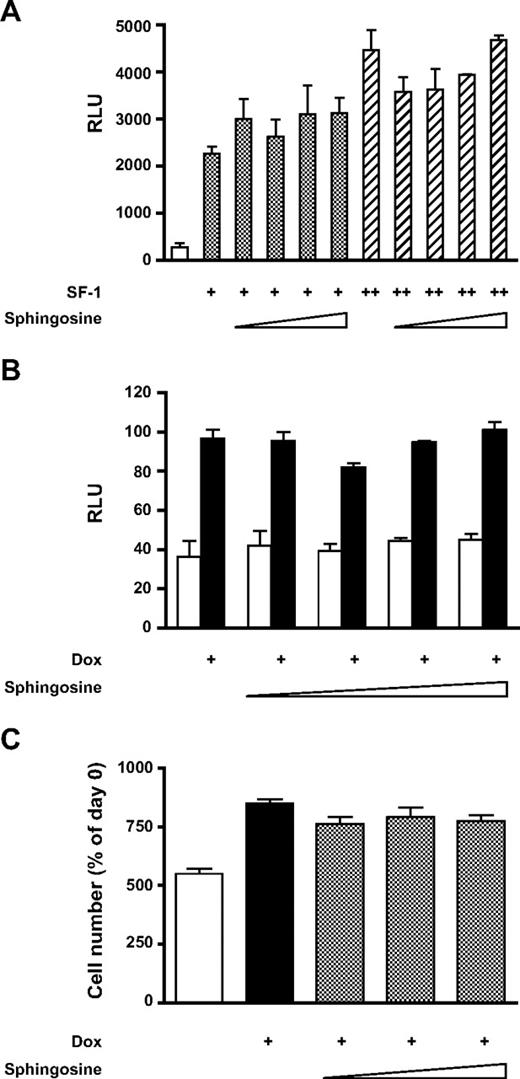
Recently, phospholipids have been shown to occupy the ligand-binding pocket in SF-1 and in the related nuclear receptor LRH-1 (30, 46–48). Mutagenesis of residues predicted to interact with phospholipids significantly reduces interaction with coactivators and transactivation activity of both NR5 proteins. These findings suggest that phospholipid signal transduction pathways may modulate SF-1-dependent gene expression. On the other hand, the 18-carbon amino alcohol sphingosine can antagonize transactivation of the CYP17 promoter by SF-1 and its binding to SF-1 can be displaced by phospholipids (43, 49). We then tested whether sphingosine can antagonize SF-1-stimulated expression of the FATE1 promoter and increased proliferation of H295R TR/SF-1 cells triggered by SF-1 overexpression. Different concentrations of sphingosine did not antagonize SF-1 transactivation of the FATE1 promoter in COS cells (Fig. 9A), nor did they decrease FATE1 promoter stimulation by higher SF-1 levels in doxycycline-treated H295R TR/SF-1 cells (Fig. 9B). Finally, the increase of H295R TR/SF-1 cell proliferation triggered by higher SF-1 expression was insensitive to sphingosine concentrations from 0.1–5 nm (Fig. 9C).
Sphingosine Does Not Affect Up-Regulation of FATE1 Promoter Expression and H295R Cell Proliferation by Increased SF-1 Dosage A, FATE1 promoter-luciferase was transfected in COS cells, and its expression was measured after cotransfection with SF-1 expression vector (0.25 or 0.5 μg) in the presence of solvent (dimethylsulfoxide) or of increasing concentrations of sphingosine (0.1, 1, 5, and 10 μm); B, FATE1 promoter-luciferase was transfected in H295R TR/SF-1 cells cultured in basal conditions (white bars) or treated with doxycycline (Dox; 1 μg/ml; black bars) in the presence of solvent (dimethylsulfoxide) or of increasing concentrations of sphingosine (0.1, 1, 5, and 10 μm); C, proliferation of H295R TR/SF-1 cells was measured for cells cultured in basal conditions (white bar) or treated with doxycycline (Dox; 1 μg/ml) in the presence of solvent (dimethylsulfoxide; black bar) or of increasing concentrations of sphingosine (0.1, 1, and 5 μm). Proliferation is expressed as percentage of cell number on d 0 after a 6-d culture period. RLU, Relative luciferase units.
Sf-1 Transgenic Mice Develop Adrenocortical Hyperplasia and Tumor Formation
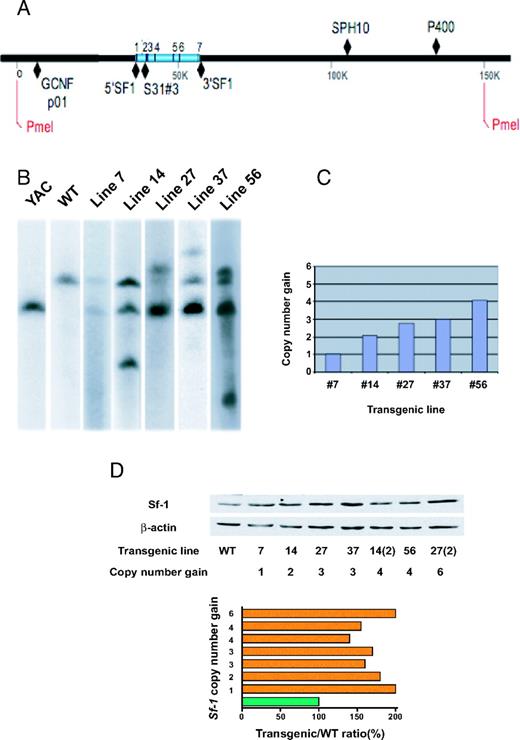
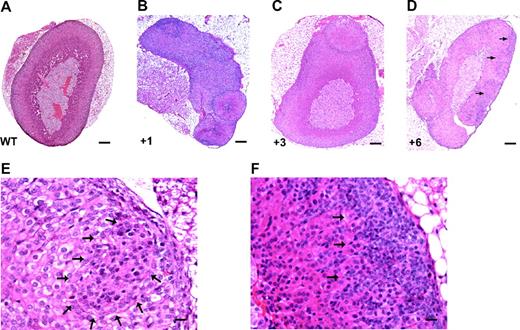
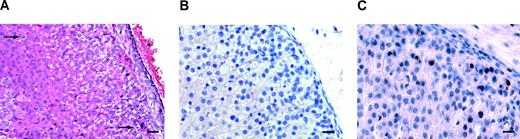
YAC TR mice bearing multiple copies of the Sf-1 (Ftz-F1; Nr5a1) gene have been described (50). Several lines of mice have been generated carrying different Sf-1 copy numbers (Fig. 10, A–C), and Sf-1 protein levels are up-regulated in the adrenal gland of Sf-1 TR mice in a manner that is not directly proportional to copy number (Fig. 10D). Sf-1 TR mice rapidly develop adrenocortical nodular hyperplasia with subsequent tumor formation, with a complete penetrance of the tumor phenotype (Fig. 11 and Tables 2 and 3). The first histological lesions in young animals are represented by groups of small, densely arranged cells that accumulate beneath the adrenal capsule (Fig. 11, E and F). Mitoses are detectable in the adrenal cortex of young Sf-1 TR mice, whereas they are never present in the adrenal cortex of WT littermates (Fig. 12A). Adrenocortical cells in TR mice are labeled by the Ki-67 proliferation marker (Fig. 12, B and C). In older animals, ACT may also spread into surrounding tissues (data not shown).
Characterization of Sf-1 TR Mouse Lines A, AA, A large restriction fragment containing 156 kb of Sf-1 encoding and flanking sequences was generated by PmeI digestion and analyzed by Southern blotting using several probes located across the fragment. The positions of Sf-1 exons, PmeI restriction sites, and probes GCNFp01, 5′SF1, S31#3, 3′SF1, SPH10, and P400 are indicated. B, Evaluation of TR Sf-1 copy number in various TR mouse lines. Genomic DNA fragments in different TR mouse lines were revealed by radiolabeled 5′SF1 probe. C, Numbers of TR copies in lines 7, 14, 27, 37, and 56 were quantified by densitometry taking the signal derived from the endogenous mouse Sf-1 gene as a reference. D, Immunoblot showing Sf-1 protein levels in adrenals of mice from TR mice from lines 7, 14, 27, 37, homozygous 14, heterozygote 56, and homozygote 27. Copy number gain is indicated for each TR line. Data were quantified (histogram) by densitometry after normalization by β-actin levels. Data are expressed as TR/WT Sf-1 ratio.
Sf-1 Overexpression Causes Adrenocortical Nodule and Tumor Formation in Mice A, Normal adrenal from a WT mouse; B–D, adrenocortical hyperplasia in Sf-1 TR mice from different TR lines (7, 14, 37 ), with the number of TR Sf-1 copies indicated for each sample; E and F dysplastic lesions in the adrenal cortex of a 10-d-old Sf-1 TR mouse harboring four TR Sf-1 copies. In D–F, arrows indicate areas occupied by dysplastic, abnormally organized cell clusters lying in the subcapsular adrenal region. Magnification, ×10 magnification (A–D; bar, 200 μm) and ×40 (E and F; bar, 20 μm).
Cell Proliferation Is Increased in the Adrenal Cortex of Sf-1 TR Mice A, Mitoses (arrows) are detectable in the adrenal cortex of a homozygous 10-d-old mouse from line 14; B and, Ki-67 staining of the adrenals of a 21-d-old WT (B) and TR (C) mouse bearing eight Sf-1 copies. Magnification, ×40 magnification; bar, 20 μm.
Statistics of ACT Incidence in Sf-1 TR Mice
| Line . | No. of Animals Examined . | No. of Animals with ACT . |
|---|---|---|
| 56 | 6 | 6 |
| 37 | 3 | 3 |
| 27 | 1 | 1 |
| 14 | 9 | 9 |
| 7 | 2 | 2 |
| WT | 7 | 0 |
| Line . | No. of Animals Examined . | No. of Animals with ACT . |
|---|---|---|
| 56 | 6 | 6 |
| 37 | 3 | 3 |
| 27 | 1 | 1 |
| 14 | 9 | 9 |
| 7 | 2 | 2 |
| WT | 7 | 0 |
Statistics of ACT Incidence in Sf-1 TR Mice
| Line . | No. of Animals Examined . | No. of Animals with ACT . |
|---|---|---|
| 56 | 6 | 6 |
| 37 | 3 | 3 |
| 27 | 1 | 1 |
| 14 | 9 | 9 |
| 7 | 2 | 2 |
| WT | 7 | 0 |
| Line . | No. of Animals Examined . | No. of Animals with ACT . |
|---|---|---|
| 56 | 6 | 6 |
| 37 | 3 | 3 |
| 27 | 1 | 1 |
| 14 | 9 | 9 |
| 7 | 2 | 2 |
| WT | 7 | 0 |
Pathological Features of ACT in Sf-1 TR Mice
| Line . | Transgene Genotype . | Extra Copies of Sf-1 . | Animal no. . | Age . | Sex . | Adrenocortical Nodule . | Metastasis . |
|---|---|---|---|---|---|---|---|
| 56 | +/+ | 8 | 561 | 21 d | F | + | |
| 56 | +/− | 4 | 10233 | 6 months | M | + | |
| 56 | +/− | 4 | 10231 | 3 months | F | Kidney, ovary | |
| 37 | +/+ | 6 | 10932 | 3.5 months | F | + | |
| 37 | +/− | 3 | 9447 | 3 months | M | + | |
| 27 | +/+ | 6 | 11076 | 25 d | F | + | |
| 14 | +/+ | 4 | 9 | 10 d | F | + | |
| 14 | +/+ | 4 | 16 | 10 d | M | + | |
| 14 | +/− | 2 | 5921 | 9 months | M | + | |
| 14 | +/+ | 4 | 6068 | 9 months | M | + | |
| 14 | +/− | 2 | 7425 | 12 months | M | Bilateral | |
| 14 | +/− | 2 | 7111 | 12 months | F | + | |
| 14 | +/− | 2 | 7481 | 12 months | F | Bilateral | |
| 14 | +/− | 2 | 7055 | 12 months | F | + | |
| 14 | +/− | 2 | 7110 | 12 months | F | + |
| Line . | Transgene Genotype . | Extra Copies of Sf-1 . | Animal no. . | Age . | Sex . | Adrenocortical Nodule . | Metastasis . |
|---|---|---|---|---|---|---|---|
| 56 | +/+ | 8 | 561 | 21 d | F | + | |
| 56 | +/− | 4 | 10233 | 6 months | M | + | |
| 56 | +/− | 4 | 10231 | 3 months | F | Kidney, ovary | |
| 37 | +/+ | 6 | 10932 | 3.5 months | F | + | |
| 37 | +/− | 3 | 9447 | 3 months | M | + | |
| 27 | +/+ | 6 | 11076 | 25 d | F | + | |
| 14 | +/+ | 4 | 9 | 10 d | F | + | |
| 14 | +/+ | 4 | 16 | 10 d | M | + | |
| 14 | +/− | 2 | 5921 | 9 months | M | + | |
| 14 | +/+ | 4 | 6068 | 9 months | M | + | |
| 14 | +/− | 2 | 7425 | 12 months | M | Bilateral | |
| 14 | +/− | 2 | 7111 | 12 months | F | + | |
| 14 | +/− | 2 | 7481 | 12 months | F | Bilateral | |
| 14 | +/− | 2 | 7055 | 12 months | F | + | |
| 14 | +/− | 2 | 7110 | 12 months | F | + |
F, Female; M, male.
Pathological Features of ACT in Sf-1 TR Mice
| Line . | Transgene Genotype . | Extra Copies of Sf-1 . | Animal no. . | Age . | Sex . | Adrenocortical Nodule . | Metastasis . |
|---|---|---|---|---|---|---|---|
| 56 | +/+ | 8 | 561 | 21 d | F | + | |
| 56 | +/− | 4 | 10233 | 6 months | M | + | |
| 56 | +/− | 4 | 10231 | 3 months | F | Kidney, ovary | |
| 37 | +/+ | 6 | 10932 | 3.5 months | F | + | |
| 37 | +/− | 3 | 9447 | 3 months | M | + | |
| 27 | +/+ | 6 | 11076 | 25 d | F | + | |
| 14 | +/+ | 4 | 9 | 10 d | F | + | |
| 14 | +/+ | 4 | 16 | 10 d | M | + | |
| 14 | +/− | 2 | 5921 | 9 months | M | + | |
| 14 | +/+ | 4 | 6068 | 9 months | M | + | |
| 14 | +/− | 2 | 7425 | 12 months | M | Bilateral | |
| 14 | +/− | 2 | 7111 | 12 months | F | + | |
| 14 | +/− | 2 | 7481 | 12 months | F | Bilateral | |
| 14 | +/− | 2 | 7055 | 12 months | F | + | |
| 14 | +/− | 2 | 7110 | 12 months | F | + |
| Line . | Transgene Genotype . | Extra Copies of Sf-1 . | Animal no. . | Age . | Sex . | Adrenocortical Nodule . | Metastasis . |
|---|---|---|---|---|---|---|---|
| 56 | +/+ | 8 | 561 | 21 d | F | + | |
| 56 | +/− | 4 | 10233 | 6 months | M | + | |
| 56 | +/− | 4 | 10231 | 3 months | F | Kidney, ovary | |
| 37 | +/+ | 6 | 10932 | 3.5 months | F | + | |
| 37 | +/− | 3 | 9447 | 3 months | M | + | |
| 27 | +/+ | 6 | 11076 | 25 d | F | + | |
| 14 | +/+ | 4 | 9 | 10 d | F | + | |
| 14 | +/+ | 4 | 16 | 10 d | M | + | |
| 14 | +/− | 2 | 5921 | 9 months | M | + | |
| 14 | +/+ | 4 | 6068 | 9 months | M | + | |
| 14 | +/− | 2 | 7425 | 12 months | M | Bilateral | |
| 14 | +/− | 2 | 7111 | 12 months | F | + | |
| 14 | +/− | 2 | 7481 | 12 months | F | Bilateral | |
| 14 | +/− | 2 | 7055 | 12 months | F | + | |
| 14 | +/− | 2 | 7110 | 12 months | F | + |
F, Female; M, male.
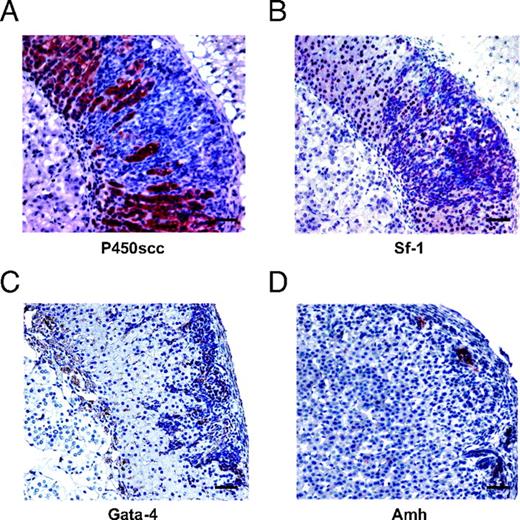
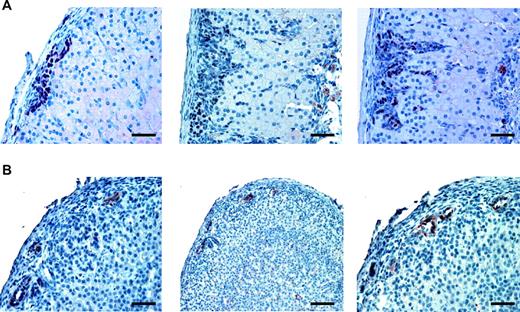
Neoplastic Cells in Sf-1 Transgenic Mice Adrenals Have a Gonadal Cell Phenotype and Express Activated Signal Transducer and Activator of Transcription 3 (Stat3)
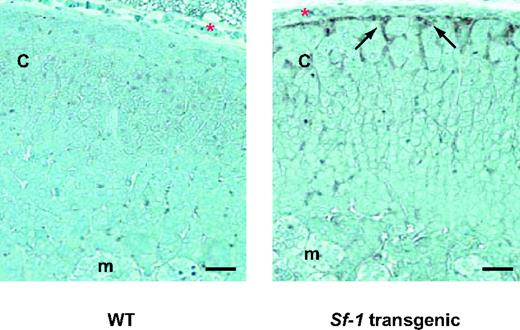
The histological appearance of proliferating adrenocortical cells in Sf-1 TR mice and their subcapsular localization strikingly resemble the pattern of proliferative adrenocortical lesions that develop in specific strains of gonadectomized mice (9). Gonadal markers are expressed in adrenal tumors arising in the above mentioned strains of gonadectomized mice. Similarly, hyperproliferative cells in the adrenal cortex of Sf-1 TR mice are negative for expression of the steroidogenic enzyme P450scc, whereas they express Sf-1, Amh, and Gata-4 (GATA binding protein 4) (Figs. 13 and 14). Adrenal glands of five of nine Sf-1 TR mice examined were positive for either anti-Mullerian hormone (Amh) or Gata-4 by immunohistochemistry, whereas seven of 17 Sf-1 TR adrenals were positive for Gata-4 expression (supplemental Table 2; it was not possible to measure Amh levels by immunoblotting because the available antibody was not suitable for use in this technique). As expected, WT mice adrenals were always negative for Amh (immunohistochemistry) and Gata-4 (immunohistochemistry and immunoblotting). Amh staining is distributed in distinct foci inside neoplastic areas, whereas Gata-4 is expressed in groups of subcapsular cells extending in a columnar pattern inside the structure of the adrenal cortex (Figs. 13 and 14). Remarkably, gonadotropin levels are normal in Sf-1 TR mice (Table 4). Microarray analysis of gene expression profiles in the adrenals of Sf-1 TR mice revealed a higher expression of transcription factor Stat3 compared with WT adrenals Also, genes encoding proteins with roles in cell adhesion to the extracellular matrix (Col23a1, Col5a3, Chodl, Vnn1, Gja1, Ly6d, and Pvrl2) and immune response (C1qg, Vnn1, and Bcl6) were differentially regulated in Sf-1 TR adrenals compared with normal mice (supplemental Fig. 2). Y705-phosphorylated Stat3 was detected in the adrenal cortex of 3-wk-old Sf-1 TR mice, localizing in a thin rim of cells lying beneath the adrenal capsule, whereas adrenals from age-matched WT mice (Fig. 15) and 3- to 3.5-month-old Sf-1 TR mice (not shown) were negative for phospho-Stat3 immunostaining.
Hyperplastic Lesions in Sf-1 TR Mice Express Gonadal Markers Hyperplastic nodules in the adrenal cortex are negative for P450scc expression (A), whereas they express Sf-1 (B), focal Gata-4 (C), and Amh (D). Magnification, ×25; bar, 50 μm.
More Images of Gata-4 and Amh Expression in Sf-1 TR Mice Adrenals A, Clusters of Gata-4-positive cells extend in a columnar pattern inside the adrenal cortex; B, images of focal Amh staining. Magnification, ×25; bar, 50 μm.
Activated Stat3 Expression in Sf-1 TR Mice Adrenals Y705-phosphorylated Stat3 immunoreactivity (black arrows) is present in a thin rim of subcapsular adrenocortical cells in a 21-d-old animal from line 56 (right panel), whereas it is absent in a WT littermate (left panel). c, Adrenal cortex; m, adrenal medulla. Red asterisk, adrenal capsule. Arrows indicate Y705-phosphorylated Stat3-positive cells. Magnification, ×25; bar, 50 μm.
Plasma Gonadotropin Levels in Sf-1 TR and WT Mice
| Genotype . | Sex . | Age . | n . | FSH (ng/ml) . | LH (ng/ml) . |
|---|---|---|---|---|---|
| YAC | Male | 4–6 months | 6 | 27.3 ± 10.1 | 0.34 ± 0.22 |
| WT | Male | 4–6 months | 7 | 40.6 ± 13.9 | 0.14 ± 0.02 |
| YAC | Female | 4–6 months | 14 | 5.4 ± 2.6 | 0.23 ± 0.18 |
| WT | Female | 4–6 months | 5 | 3.5 ± 0.9 | 0.25 ± 0.13 |
| YAC | Male | 25 d | 4 | 29.9 ± 4.0 | 0.18 ± 0.15 |
| WT | Male | 25 d | 2 | 44.2 ± 4.8 | 0.48 ± 0.38 |
| YAC | Female | 25 d | 10 | 4.2 ± 1.9 | 0.25 ± 0.22 |
| WT | Female | 25 d | 3 | 4.3 ± 1.7 | 0.12 ± 0.04 |
| Genotype . | Sex . | Age . | n . | FSH (ng/ml) . | LH (ng/ml) . |
|---|---|---|---|---|---|
| YAC | Male | 4–6 months | 6 | 27.3 ± 10.1 | 0.34 ± 0.22 |
| WT | Male | 4–6 months | 7 | 40.6 ± 13.9 | 0.14 ± 0.02 |
| YAC | Female | 4–6 months | 14 | 5.4 ± 2.6 | 0.23 ± 0.18 |
| WT | Female | 4–6 months | 5 | 3.5 ± 0.9 | 0.25 ± 0.13 |
| YAC | Male | 25 d | 4 | 29.9 ± 4.0 | 0.18 ± 0.15 |
| WT | Male | 25 d | 2 | 44.2 ± 4.8 | 0.48 ± 0.38 |
| YAC | Female | 25 d | 10 | 4.2 ± 1.9 | 0.25 ± 0.22 |
| WT | Female | 25 d | 3 | 4.3 ± 1.7 | 0.12 ± 0.04 |
Plasma Gonadotropin Levels in Sf-1 TR and WT Mice
| Genotype . | Sex . | Age . | n . | FSH (ng/ml) . | LH (ng/ml) . |
|---|---|---|---|---|---|
| YAC | Male | 4–6 months | 6 | 27.3 ± 10.1 | 0.34 ± 0.22 |
| WT | Male | 4–6 months | 7 | 40.6 ± 13.9 | 0.14 ± 0.02 |
| YAC | Female | 4–6 months | 14 | 5.4 ± 2.6 | 0.23 ± 0.18 |
| WT | Female | 4–6 months | 5 | 3.5 ± 0.9 | 0.25 ± 0.13 |
| YAC | Male | 25 d | 4 | 29.9 ± 4.0 | 0.18 ± 0.15 |
| WT | Male | 25 d | 2 | 44.2 ± 4.8 | 0.48 ± 0.38 |
| YAC | Female | 25 d | 10 | 4.2 ± 1.9 | 0.25 ± 0.22 |
| WT | Female | 25 d | 3 | 4.3 ± 1.7 | 0.12 ± 0.04 |
| Genotype . | Sex . | Age . | n . | FSH (ng/ml) . | LH (ng/ml) . |
|---|---|---|---|---|---|
| YAC | Male | 4–6 months | 6 | 27.3 ± 10.1 | 0.34 ± 0.22 |
| WT | Male | 4–6 months | 7 | 40.6 ± 13.9 | 0.14 ± 0.02 |
| YAC | Female | 4–6 months | 14 | 5.4 ± 2.6 | 0.23 ± 0.18 |
| WT | Female | 4–6 months | 5 | 3.5 ± 0.9 | 0.25 ± 0.13 |
| YAC | Male | 25 d | 4 | 29.9 ± 4.0 | 0.18 ± 0.15 |
| WT | Male | 25 d | 2 | 44.2 ± 4.8 | 0.48 ± 0.38 |
| YAC | Female | 25 d | 10 | 4.2 ± 1.9 | 0.25 ± 0.22 |
| WT | Female | 25 d | 3 | 4.3 ± 1.7 | 0.12 ± 0.04 |
DISCUSSION
Here we have shown that an increased dosage of the nuclear hormone receptor SF-1 increases proliferation and modulates the pattern of steroid secretion in human adrenocortical cells and triggers adrenal tumor formation in mice. These findings are important to understand the pathogenesis of human childhood ACT, where the SF-1 gene is amplified (27) and overexpressed (28). Our results show that SF-1 dosage is a critical factor for the control of adrenocortical cell growth not only during development, when SF-1 deficiency causes adrenal insufficiency in both mice and humans (20–26), but also for the process of adrenocortical tumorigenesis. Other situations are known where transcription factor dosage is a critical determinant of its biological action, for example in the case of Oct-3/4 dosage in mouse embryonic stem cells (51).
The SF-1 effect on adrenocortical cell proliferation requires the factor to be transcriptionally active through its AF-2 domain and can be dissected into both an activation of cell proliferation and a reduction in apoptosis. Remarkably, the related nuclear receptor LRH-1 (NR5A2) has also been shown to activate intestinal cell proliferation (52). The role of androgen receptor and estrogen receptor-α amplification and overexpression in prostate and breast cancer progression is also well known (53, 54). A remarkable difference between these situations is that SF-1 overexpression in ACT appears to be a frequent event that is associated with transformation but not with malignancy. In contrast, Sf-1 was found to inhibit proliferation in rat ovarian surface epithelial cells (55). These data suggest that the SF-1 role in regulating cell proliferation can be different according to species and tissue.
Gene expression profiling revealed that an increase of SF-1 dosage in human tumor adrenocortical cells modulates a coordinated set of transcript which are significantly enriched in genes involved in lipid and steroid metabolism, apoptosis, cell cycle progression, and cell adhesion. Remarkably, modulation of the expression of several genes whose products have a function in the adhesion to the extracellular matrix is also found by analysis of differentially regulated genes in Sf-1 TR mouse adrenals. Regulation by SF-1 dosage of this category of genes represents a common theme between our cellular and animal models and opens new perspectives in the understanding of the biological processes regulated by SF-1. On the other hand, genes involved in immune response are also found differentially expressed in Sf-1 TR mouse adrenals. These findings are particularly intriguing, because the expression of some immune response genes was found to be able to distinguish adenomas from carcinomas by our microarray study on childhood ACT (36). These results suggest that the immune response can modulate ACT growth and malignancy, by mechanisms still unknown, and that SF-1 dosage may represent an important factor in its regulation. Interestingly, one gene that we found overexpressed in Sf-1 TR mouse adrenals and that plays a role in both cell adhesion and immune response is vanin-1 (Vnn1) (56, 57). It has been shown that Sf-1 and Sox9 cooperate to regulate Vnn1 expression in the developing mouse testis (58). These data support a mechanism of direct regulation of Vnn1 by Sf-1 dosage in the adrenal gland of Sf-1 TR mice.
FATE1 has been implicated in regulating proliferation of hepatocarcinoma cells (42). An increased SF-1 dosage increases its recruitment to the FATE1 promoter and modulates the recruitment of specific cofactors, increasing the association of CBP with the promoter and decreasing recruitment of the HDAC1 histone deacetylase (Fig. 8). These data are consistent with previous results that showed that a dynamic interplay between cofactors is necessary for regulation of gene expression by SF-1 (42–44). Conversely, sphingosine, which was reported to inhibit SF-1-dependent activation of the CYP17 gene (42, 48), had no effect on SF-1-dosage-regulated FATE1 gene and dosage-dependent increase of H295R cell proliferation. These results, together with the blunted but still detectable effect of the A433W SF-1 mutant, which has impaired phospholipid binding properties (30, 45–47), on H295R proliferation, suggest that the effect of SF-1 dosage on H295R gene expression and proliferation is at least partially independent from its phospholipid binding activity.
One surprising result of our study is the selective steroidogenic enzyme modulation by increased levels of SF-1 and the resulting pattern of steroids secreted, with reduction of cortisol and aldosterone and maintenance of DHEA-S production. Previous observations made with the use of a dominant-negative SF-1 mutant devoid of a large part of its C-terminal domain had suggested that SF-1 regulates basal and inducible transcription of CYP17, CYP21A2, and CYP11B1 in H295R cells (59). It may seem paradoxical that HSD3B2 and CYP21A2 genes are down-regulated consequently to SF-1 overexpression, because their promoters harbor SF-1 binding sites that have been shown to be functional in transient transfection experiments (60, 61). On the other hand, definitive evidence that this factor is an essential regulator of steroidogenic enzyme expression in vivo is still lacking because of the adrenogonadal agenesia present in Sf-1 null mice (20, 21). We suggest that an increased SF-1 dosage in H295R cells reinforces their differentiation toward the fetal adrenal phenotype, characterized by production of high levels of DHEA-S, whereas it inhibits differentiation toward an adult adrenal cell that produces glucocorticoids and mineralocorticoids. This is also consistent with the observed up-regulation of steroid sulfotransferase 2A1, the enzyme responsible for sulfonation of hydroxysteroids in the human adrenal, upon SF-1 overexpression (62) (supplemental Table 1). On the other hand, it is remarkable that the isolated overexpression of SF-1 in adrenocortical cells suffices to phenocopy several features of childhood ACT, which display a similar down-regulation of HSD3B2 and CYP21A2 and whose general pattern of gene expression is highly correlated to the fetal adrenal pattern (36). Moreover, NOV/CCN3, a proapoptotic factor for adrenocortical cells strongly down-regulated by increased SF-1 dosage in our cellular system, is also significantly down-regulated in childhood ACT (36, 37). Taken together, these findings show the relevance of our system as a cellular model for human childhood ACT.
In mice, increased Sf-1 dosage is sufficient to trigger adrenocortical tumorigenesis. At variance with the fetal adrenal phenotype of human childhood tumors, most tumors arising in mice TR for Sf-1 have a phenotype resembling gonadal cell tumors. They are characterized by expression of gonadal markers such as Amh and Gata-4, absence of steroidogenic enzymes such as P450scc, and expression of activated Stat3. This phenotype is reminiscent of ACT occurring in gonadectomized animals belonging to particular laboratory strains (9). Those tumors are thought to be derived from pluripotent adrenogonadal precursor cells lying beneath the outer adrenal connective capsule, which in the susceptible strains still have the potential to differentiate into cells of gonadal stroma phenotype (14–16). In rare cases, human ACT can express LH receptors, in cases of both cortisol- and aldosterone-producing neoplasms, and GATA-4 (63–66). These data, together with the relatively common finding of adrenal rests in the testes of male patients with congenital adrenal hyperplasia (67), suggest that also in humans, pluripotent precursor cells can be found in both adrenals and gonads. Alternatively, tumor cells in these organs can dedifferentiate to acquire either an adrenal or a gonadal phenotype. Remarkably, susceptible mice strains all possess the Sf-1S172 allele. Although S172 Sf-1 does not have an altered transactivation activity by itself, it is associated with lower steroid production in mouse Y1 adrenocortical cells (68). It is tempting to speculate that the Sf-1S172 allele may be associated with high Sf-1 expression dependent on high gonadotropin levels and predisposes to the development of ACT.
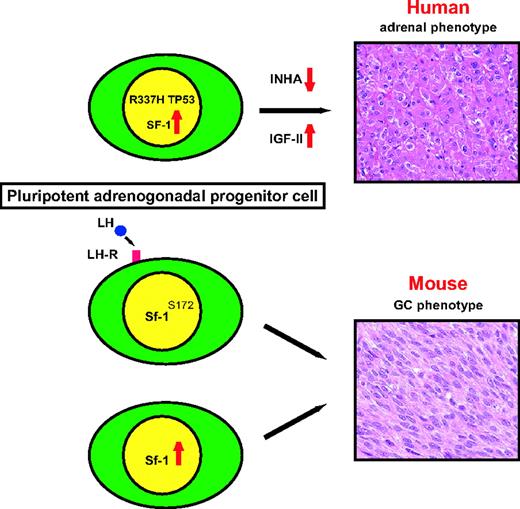
A model for the SF-1 role in human and mouse adrenocortical tumorigenesis is shown in Fig. 16. In childhood ACT, SF-1 is amplified and overexpressed in adrenocortical cells harboring a germline TP53 mutation (probably determining a defect in apoptosis). This triggers increased proliferation and adenoma formation. These tumors have a fetal adrenal phenotype, producing high amounts of androgenic steroids. Other genetic abnormalities, e.g. inhibin-α gene mutations (69) and growth factor and growth factor receptor overexpression (35), may ensue, accelerate cell proliferation, and in some cases, produce malignancy. Conversely, in mice, high levels of Sf-1 induced by TR overexpression or genetically determined in susceptible strains (bearing the Sf-1S172 allele) under the control of gonadotropins, may trigger proliferation of pluripotent cells lying in the subcapsular region of the adrenal cortex and tumor formation. Interestingly, our studies revealed that Y705-phosphorylated Stat3 is expressed in the subcapsular region of the adrenal cortex in young Sf-1 TR mice. Tyrosine phosphorylation of Stat3 is an important requisite for the oncogenic properties of this transcription factor (70) and is probably the result of the activation of local cytokine and inflammatory pathways during the process of transformation in Sf-1 TR adrenocortical cells.
Model Showing the Role of Increased SF-1 Dosage in Human and Mouse Adrenocortical Tumorigenesis In children, in the presence of a germline TP53 mutation and loss of heterozygosity, increased SF-1 dosage augments proliferation and inhibits apoptosis of adrenocortical cells around the period of physiological fetal adrenal regression. Concomitantly with other genetic lesions (e.g. inhibin-α mutations, IGF-II overexpression), ACT with a fetal adrenal phenotype may ensue. In mice, pluripotent adrenogonadal progenitor cells are present in the subcapsular region of the adrenal cortex. Susceptible strains harboring the Sf-1S172 allele, which may predispose to increased Sf-1 expression, or lacking inhibin-α develop gonadal-type cell tumors in the adrenal cortex under the control of pituitary LH. The same effect is produced by increased Sf-1 levels in the C57B6 background in the absence of elevated gonadotropin levels (our study). GC, Gonadal-like cells; INHA, inhibin-α.
Although the difference in tumor phenotypes underlies the profound developmental and physiopathological differences between human childhood ACT and ACT in mice, in both species, SF-1 appears to play an important pathogenetic role. In the light of these findings, a new hope for therapeutic approaches in childhood ACT (71) may come from drugs targeting SF-1 transcriptional activity.
In ACT of adults, published studies have reported varying levels of SF-1 expression. Some reports have shown similar levels of SF-1 mRNA expression in cortisol-producing adenomas and normal adrenals (72, 73) and between adenomas and carcinomas (74), whereas others have found increased expression of SF-1 mRNA in both aldosterone- and cortisol-producing adenomas (75) or reduced SF-1 levels in carcinomas, as compared with adenomas, as a probable sign of dedifferentiation (76). A possible confounding factor is that these reports, except the one by Sasano et al. (72) that studied a limited number of cases, measured SF-1 mRNA in tissues, whose levels are not always directly correlated to protein abundance. For this reason, future studies are required to assess whether SF-1 expression is increased at the protein level in at least a subset of adult ACT and contributes to their pathogenetic mechanisms.
MATERIALS AND METHODS
Cell Culture and Generation of H295R Clones
H295R cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM/F-12 supplemented with 2% NuSerum (Becton Dickinson, Le-Pont-de-Claix, France), 1% ITS Plus (Becton Dickinson), and antibiotics. For generation of SF-1-inducible H295R cell clones, we employed the T-REx system (Invitrogen, Abingdon, UK). Briefly, cells were transfected with the expression plasmid (pcDNA 6/TR) encoding the Tet repressor, and clones were selected by blasticidin (Cayla-InvivoGen, Cayla InvivoGen, Toulouse, France; 5 μg/ml) treatment. After transient transfection with the reporter plasmid pcDNA4/TO/lacZ, one clone (H295R TR cells) was selected based on the highest ratio between β-galactosidase activities in the presence and in the absence of doxycycline. This clone was further transfected with the pcDNA4/TO/SF-1 plasmid, where the human SF-1 cDNA has been inserted into the HindIII-BamHI sites of pcDNA4/TO. Further antibiotic selection with zeocin (Cayla-InvivoGen; 100 μg/ml) was realized to isolate clones overexpressing SF-1 in a doxycycline-inducible fashion.
Site-Directed Mutagenesis
The S203A, L451A/L452A (AF-2mut), and A433W mutations were introduced in the SF-1 cDNA by site-directed mutagenesis, using the QuikChange kit (Stratagene, La Jolla, CA). Mutagenic primers sequences are available on request.
Proliferation and Apoptosis Assays
BrdU labeling index and percentage of apoptotic cells were measured in H295R TR/SF-1 WT cells in basal conditions or after a 72-h doxycycline (1 μg/ml) treatment using the fluorescent in situ cell proliferation and the in situ cell death detection kits (Roche, Meylan, France), following the manufacturer’s protocol.
SF-1 Knockdown
H295R cells were seeded in 24-well culture plates in triplicate at the density of 40,000 cells per well in 1 ml complete medium, and 24 h later, each well was transfected with 50 nm of either one of two SF-1-specific Stealth siRNA (CCCGCAACAACCUGCUCAUCGAAAU and AGAGCCAGAGCUGCAAGAUCGACAA; Invitrogen) or with a control siRNA oligo (medium GC; Invitrogen) using the jetSI-ENDO transfection reagent following the manufacturer’s protocol. siRNA transfection was repeated 72 h later. Cells were harvested for counting and protein extraction 48 h after the second transfection.
EMSA
EMSA was performed as described (77), except that protein-DNA complexes were separated on a 4% polyacrylamide gel in 0.25× Tris-borate-EDTA. Sequences of double-stranded DNA oligonucleotides used as probes or as competitors were GTACTGTGTCCAAGGCCTCAGCCTAGAGAGG (FATE1 promoter WT consensus SF-1 binding site), GTACTGTGTCCAATTCCTCAGCCTAGAGAGG (FATE1 promoter mutated SF-1 binding site); GAGAAGGCCACTGACCTAGCCAGCAAGATAC (FATE1 promoter WT nuclear receptor half-site), and GAGAAGGCCACTGAAATAGCCAGCAAGATAC (FATE1 promoter mutated nuclear receptor half-site). Mutated nucleotides in the sequences are underlined. Commercial anti SF-1 antibody (Upstate Biotechnology, Lake Placid, NY) was used for supershift.
ChIP
ChIP was performed using the ChIP-IT kit (Active Motif, Rixensart, Belgium), following the manufacturer’s protocol. Antibodies used for ChIP were nonimmune rabbit IgG (Abcam, Cambridge, UK), anti-SF-1 (Upstate), anti-SRC-1 (Upstate), anti-CBP (Abcam), anti-GCN5 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-HDAC1 (Upstate). Sequences of primers used for PCR amplification of immunoprecipitated chromatin were FATE1 promoter 5′ primer, TAGCAGAGGAGAAGGCCACT; FATE1 promoter 3′ primer, GCTATGGCTAAGGATGCACA; FATE1 intron 2 5′ primer, ATGGGGTCTCCCTAATACCC; and FATE1 intron 2 3′ primer, TAGGAACTTTGCCACTGCAC.
Transient Transfection Assays
The −906/+1 FATE1 promoter fragment was cloned by PCR using H295R genomic DNA as a template. Primers used were 5′ primer, GGGGTACCGGCTTATGCGATTGTGGGGCTG, and 3′ primer, GGCTCGAGTTCCGGAAACAGGAGCTCAAGG.
The FATE1 promoter fragment was cloned in the KpnI-XhoI sites of the pGL2 luciferase vector (Promega, Charbonnières, France). Transient transfections in COS and H295R TR/SF-1 cells were performed as described (37). In some experiments, d-erythrosphingosine (Avanti Polar Lipids, Alabaster, AL) was added in the culture medium 24 h after beginning of transfection.
Immunoblots
Protein extracts were prepared by homogenization of cells and tissues in Laemmli buffer [50 mm Tris-HCl (pH 6.8), 50% glycerol, 2% SDS, 0.02% bromophenol blue] containing 5% β-mercaptoethanol. For the FATE1 immunoblot, heavy membrane fractions were prepared as described (33). Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Immunoblot was performed using a chemiluminescence system for protein detection (Amersham, Les Ulis, France). Primary antibodies used were rabbit polyclonal anti-Ad4BP/SF-1 (gift of K. Morohashi); anti-FATE1 antiserum produced in rabbit using simultaneous immunization against two peptides, amino acids 2–16 AGGPPNTKAEMEMSLC and amino acids 73–87 CTATRPKKMGSQLPKPRM coupled to keyhole limpet hemocyanin (Eurogentec, Seraing, Belgium); and mouse monoclonals anti-β-tubulin (Sigma Chemical Co., Saint Quentin Fallavier, France) and anti-β-actin (Sigma).
Steroid Assays
Aldosterone and DHEA-S levels in culture supernatants were measured by specific competitive RIA (Immunotech, Marseille, France). Cortisol was measured by a chemiluminescent assay (Advia Centaur, Bayer, Puteaux, France).
Microarrays and Data Analysis
Microarray experiments were performed as described (78). See supplemental text, supplemental Table 1, and supplemental Fig. 4.
qRT-PCR
qRT-PCR for transcript quantification was performed using the SYBR Green I dye assay on a GeneAmp 5700 (Perkin-Elmer, Norwalk, CT) instrument using TBP as a reference transcript. Primer sequences were designed using the Primer Express software and are shown in supplemental Table 3. Results are expressed using the ΔΔCt method (79).
TR Mice
TR mice lines harboring YAC clones containing the rat Ftz-F1 locus were generated as described (50). The number of TR copies in each mouse line was measured by Southern blotting using six different probes located across the TR Sf-1 locus: GCNFp01, 5′SF1, S31#3, 3′SF1, SPH10, and P400 (indicated on the scheme in Fig. 10). Sf-1 protein levels were measured by immunoblot using the anti-Sf-1 antibody and quantified by densitometry.
Histology and Immunohistochemistry
Histology and immunohistochemistry were performed using standard procedures, as described (50). Antibodies used for immunohistochemistry were anti-Ki-67 (1:50; Dako, Glostrup, Denmark), anti P450scc (1:500; generously provided by M. J. Soares), anti-Sf-1 (1:2000; generously provided by K. Morohashi), anti-Gata-4 (1:1000; Santa Cruz Biotechnology), anti-Amh (1:500; Santa Cruz Biotechnology), and anti-Y705 phospho-Stat3 (1:100; Cell Signaling Technology, Beverly, MA).
Acknowledgments
We thank B. Heller and the Institute of Genetics and Molecular and Cell Biology cell culture service, L. Tejada and M. Rengasamy for excellent technical assistance, J. Cazareth and M.-J. Fauvergue for technical help, R. Mengual for steroid assays, N. Guy and G. Pagès for help with animal experimentation, K. Morohashi and M. J. Soares for antibodies, University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for mouse LH sandwich immunoradiometric assay and mouse FSH RIA provided, and Dr. Dharam Ramnani (WebPathology.com) for histological images. We acknowledge the excellent support of the Nice-Sophia Antipolis Transcriptome Platform of the Marseille-Nice Genopole, where microarray experiments were performed.
This work was supported by National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grants U54-HD28934, National Cancer Institute CA63230, and CA71907 (to G.P.Z.). M.D. is a recipient of a Fondation Recherche Médicale fellowship. G.P.Z. was supported by National Institutes of Health Grant CA63230. Research in E.L.’s laboratory is supported by Centre National de la Recherche Scientifique Action Thématique Incitative sur Programme and Programme International de Coopération Scientifique grants, contrat d’interface Institut National de la Santé et de la Recherche Medicale-Centre Hospitalier de l’Université de Nice, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Comité Français d’Evaluation de la Coopération Universitaire avec le Bresil (Grant 419/03), Fondation Recherche Médicale, Princess Grace Foundation, and Association Recherche sur le Cancer (Grant 3142).
Microarray data have been deposited in the GEO database under the accession numbers GSE5911 and GSE5912.
Discosure Statement: All authors have nothing to declare.
M.D. and T.K. contributed equally to this work.
Abbreviations:
- ACT,
Adrenocortical tumors;
- Amh,
anti-Mullerian hormone;
- BrdU,
bromodeoxyuridine;
- CBP,
cAMP response element-binding protein-binding protein;
- ChIP,
chromatin immunoprecipitation;
- FATE1,
fetal and adult testis expressed-1;
- Gata-4,
GATA binding protein 4;
- GCN5,
general control nonderepressed-5;
- HDAC1,
histone deacetylase 1;
- NOV/CCN3,
nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3;
- qRT-PCR,
quantitative RT-PCR;
- SF-1,
steroidogenic factor-1;
- siRNA,
small interfering RNA;
- SRC-1,
steroid receptor coactivator-1;
- Stat3,
signal transducer and activator of transcription-3;
- TR,
transgenic;
- WT,
wild type.