Abstract
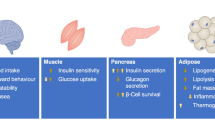
Insulin is a potent metabolic hormone essential for the maintenance of normal circulating blood glucose level in mammals. The physiologic control of glucose homeostasis results from a balance between hepatic glucose release (glycogenolysis and gluconeogenesis) and dietary glucose absorption versus skeletal muscle and adipose tissue glucose uptake and disposal. Disruption of this delicate balance either through defects in insulin secretion, liver glucose output, or peripheral tissue glucose uptake results in pathophysiological states of insulin resistance and diabetes. In particular, glucose transport into skeletal muscle and adipose tissue is the rate-limiting step in glucose metabolism and reduction in the efficiency of this process (insulin resistance) is one of the earliest predictors for the development of Type II diabetes. Importantly, recent studies have directly implicated an impairment in insulin receptor signal transduction as the prime mechanism for peripheral tissue insulin resistance. In this review, we have focused on recent developments in our understanding of the molecular mechanisms and signal transduction pathways that insulin utilizes to specifically regulate glucose uptake. The detailed understanding of these events will provide a conceptual framework for the development of new therapeutic targets to treat this chronic and debilitating disease process.
Similar content being viewed by others
References
Olson, A. L., and Pessin, J. E. (1996) Structure, function and regulation of the mammalian facilitative glucose transporter gene family. Ann. Rev. Nutr. 16, 235–256.
Kahn, B. B., and Flier, J. S. (1990) Regulation of glucose-transporter gene expression in vitro and in vivo. Diabetes Care 13, 548–564.
Bell, G. I., Fukumoto, H., Burant, C. F., Seino, S., Sivitz, W. L., and Pessin, J. E. (1991) Facilitative glucose transport proteins: structure and regulation of expression in adipose tissue. Int. J. Obesity 15, 127–132.
Klip, A., Tsakiridis, T., Marette, A., and Ortiz, P.A. (1994) Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 8, 43–53.
Charron, M. J., Katz, E. B., and Olson, A. L. (1999) GLUT4 gene regulation and manipulation. J. Biol. Chem. 274, 3253–3256.
Charron, M. J., Brosius, F. C., III, Alper, S. L., and Lodish, H. F. (1989) A glucose transport protein expressed predominately in insulin-responsive tissues. Proc. Natl. Acad. Sci. USA 86, 2535–2539.
Birnbaum, M. I. (1989) Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell 57, 305–315.
James, D. E., Brown, R., Navarro, J., and Pilch, P. F. (1988) Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333, 183–185.
James, D. E., Strube, M., and Mueckler, M. (1989) Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature 338, 83–87.
Kaestner, K. H., Christy, R. J., McLenithan, J. C., Braiterman, L. T., Cornelius, P., Pekala, P. J., et al. (1989) Sequences, tissue distribution and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 86, 3150–3154.
Fukumoto, H., Kayano, T., Buse, J. B., Edwards, Y., Pilch, P. F., Bell, G. I., et al. (1989) Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J. Biol. Chem. 264, 7776–7779.
Liu, M. L., Olson, A. L., Moye-Rowley, W. S., Buse, J. B., Bell, G. I., and Pessin, J. E. (1992) Expression and regulation of the human GLUT4/muscle-fat facilitative glucose transporter gene in transgenic mice. J. Biol. Chem. 267, 11,673–11,676.
Ikemoto, S., Thompson, K. S., Itakura, H., Lane, M. D., and Ezaki, O. (1995) Expression of an insulin-responsive glucose transporter (GLUT4) minigene in transgenic mice: effect of exercise and role in, glucose homeostasis. Proc. Natl. Acad. Sci. USA 92, 865–869.
Gibbs, E. M., Stock, J. L., McCoid, S. C., Stukenbrok, H. A., Pessin, J. E., Stevenson, R. W. et al. (1995) Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J. Clin. Invest. 95, 1512–1518.
Tsao, T. S., Burcelin, R., Katz, E. B., Huang, L., and Charron, M. J. (1996) Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes 45, 28–36.
Hansen, P. A., Gulve, E. A., Marshall, B. A., Gao, J., Pessin, J. E., Holloszy, J. O., et al. (1995) Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J. Biol. Chem. 270, 1679–1684.
Katz, E. B., Stenbit, A. E., Hatton, K., DePhino, R., and Charron, M. J. (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4 Nature 377, 151–155.
Rossetti, L., Stenbit, A. E., Chen, W., Hu, M., Barzilai, N., Katz, E. B., et al. (1997) Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene. J. Clin. Invest. 100, 1831–1839.
Abel, E. D., Peroni, O., Kim, J. K., Kim Y. B., Boss, O., Hadro, E., et al. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733.
Jhun, B. H., Rampal, A. L., Liu, H., Lachaal, M., and Jung, C. Y. (1992) Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. J. Biol. Chem. 268, 17,710–17,715.
Czech, M. P., and Buxton, J. M. (1993) Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J. Biol. Chem. 268, 9187–9190.
Satoh, S., Nishimura, H., Clark, A. E., Kozka, I. J., Vannucci, S. J., Simpson, I. A., et al (1993) Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 268, 17,820–17,829.
Yang, J., and Holman, G. D. (1993) Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J. Biol. Chem. 268, 4600–4603.
Czech, M. P. and Corvera, S. (1999) Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 274, 1865–1868.
Rea, S. and James, D. E. (1997) Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes 46, 1667–1677.
Pessin, J. E., Thurmond, D. C., Elmendorf, J. S., Coker, K. J., and Okada, S. (1999) Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! location! location! J. Biol. Chem. 274, 2593–2596.
Kandror, K. V. and Pilch, P. F. (1996) Compartmentalization of protein traffic in insulin-sensitive cells. Am. J. Physiol. 271, E1-E14.
Summers, S. A., Yin, V. P., Whiteman, E. L., Garza, L. A., Cho, H., Tuttle, R. L., et al. (1999) Signaling pathways mediating insulin-stimulated glucose transport. Ann. NY Acad. Sci. 892, 169–186.
Holman, G. D. and Cushman, S. W. (1994) Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. BioEssays 16, 753–759.
Pessin, J. E. and Saltiel, A. R. (2000) Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Invest. 106, 165–169.
Baumann, C. A. and Saltiel, A. R. (2001) Spatial compartmentalization of signal transduction in insulin action. BioEssays 23, 215–222.
Lee, J. S. and Pilch, P. F. (1994) The insulin receptor—structure, function, and signaling. Am. J. Physiol. 266, C319-C334.
White, M. and Kahn, C. (1994) The insulin signaling system. J. Biol. Chem. 269, 1–4.
Cheatham, B. and Kahn, C. R. (1995) Insulin action and the insulin signaling network. Endocr. Rev. 16, 117–142.
Virkamaki, A., Ueki, K., and Kahn, C. R. (1999) Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 103, 931–943.
White, M. F. (1998) The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Recent Prog. Horm. Res. 53, 119–138.
Liu, F. and Roth, R. A. (1998) Binding of SH2 containing proteins to the insulin receptor: a new way for modulating insulin signalling. Mol. Cell. Biochem. 182, 73–78.
Goldfine, I. D. (1987) The insulin receptor; molecular biology and transmembrane signaling. Endocr. Rev. 8, 235–255.
Taylor, S. I., Cama, A., Accili, D., Barbetti, F., Quon, M. J., De La Luz Sierra, M., et al. (1992) Mutations in the insulin receptor gene. Endocr. Rev. 13, 566–595.
Frattali, A. L. and Pessin, J. E. (1993) Molecular defects of insulin/IGF-1 receptor transmembrane signaling. Ann. NY Acad. Sci. 687, 77–89.
Czech, M. P. (1985) The nature and regulation of the insulin receptor: structure and function. Annu. Rev. Physiol. 47, 357–381.
Kahn, C. R. (1985) The molecular mechanism of insulin action. Annu. Rev. Med. 36, 429–451.
Frattali, A. L. and Pessin, J. E. (1993) Relationship between α subunit ligand occupancy and β subunit autophosphorylation in insulin/insulin-like growth factor-1 hybrid receptors. J. Biol. Chem. 268, 7393–7400.
Lee, J., O'Hare, T., Pilch, P. F., and Shoelson, S. E. (1993) Insulin receptor autophosphorylation occurs asymmetrically. J. Biol. Chem. 268, 4092–4098.
Yu, K.-T. and Czech, M. P. (1986) Tyrosine phosphorylation of insulin receptor β subunit activates the receptor tyrosine kinase in intact H-35 hepatoma cells. J. Biol. Chem. 261, 4715–4722.
White, M. F., Shoelson, S. E., Keutmann, H., and Kahn, C. R. (1988) A cascade of tyrosine autophosphorylation in the β-subunit activates the phosphotransferase of the insulin recepton. J. Biol. Chem. 263, 2969–2980.
Kohanski, R. A. and Lane, M. D. (1986) Kinetic evidence for activating and non-activating components of autophosphorylation of the insulin receptor protein kinase. Biochem. Biophys. Res. Commun. 134, 1312–1318.
Klein, H. H., Freidenberg, G. R., Kladde, M., and Olefsky, J. M. (1986) Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. J. Biol. Chem. 261, 4691–4697.
Flores-Riveros, J. R., Sibley, E., Kastelic, T., and Lane, M. D. (1989) Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. J. Biol. Chem. 264, 21,557–21,572.
White, M. F. (1997) The insulin signalling system and the IRS proteins. Diabetologia 40, S2–517.
Yenush, L. and White, M. F. (1997) The IRS-signalling system during insulin and cytokine action. BioEssays 19, 491–500.
Ogawa, W., Matozaki, T., and Kasuga, M. (1998) Role of binding proteins to IRS-1 in insulin signalling. Mol. Cell. Biochem. 182, 13–22.
White, M. F. (1998) The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182, 3–11.
Hunter, T. (2000) Sighaling-2000 and beyond. Cell 100, 113–127.
Araki, E., Lipes, M. A., Patti, M. E., Bruning, J. C., Haag, B., Johnson, R. S., et al. (1994) Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene, Nature 372, 186–190.
Tamemoto, H., Kadowaki, T., Tobe, K., Yagi, T., Sakura, H., Hayakawa, T., et al. (1994) Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372, 182–186.
Withers, D. J., Gutierrez, J. S., Towery, H., Burks, D. J., Ren, J. M., Previs, S., et al. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904.
Davis, R. J. (1995) Protein, nucleotide transcriptional regulation by MAP kinases. Mol. Reprod. Dev. 42, 459–467.
Kyriakis, J. M. and Avruch, J. (1996) Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays 18, 567–577.
Schlessinger, J. (1994) SH2/SH3 signaling proteins. Curr. Opin. Genet. Dev. 4, 25–30.
Schlessinger, J. and Bar-Sagi, D. (1994) Activation of Ras and other signaling pathways by receptor tyrosine kinases. Cold Spring Harbor Symp. Quant. Biol. 59, 173–179.
Avruch, J. (1998) Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 182, 31–48.
Yuryev, A. and Wennogle, L. P. (1998) The RAF family: an expanding network of post-translational controls and protein-protein interaction. Cell Res. 8, 81–98.
Robinson, M. J. and Cobb, M. H. (1997) Mitogen-activated protein kinase pathways. Curr. Opin. Cell. Biol. 9, 180–186.
Ouwens, D. M., Vanderzon, G. C. M., Pronk, G. J., Bos, J. L., Moller, W., Cheatham, B., et al. (1994) A mutant insulin receptor induces formation of a Shc- growth factor receptor bound protein 2 (Grb2) complex and p21 (ras)-GTP without detectable interaction of insulin receptor substrate 1 (IRS1) with Grb2—evidence for IRS1-independent p21 (ras)-GTP formation. J. Biol. Chem. 269, 33,116–33,122.
Yamauchi, K. and Pessin, J. E. (1994) Insulin receptor substrate-1 (IRS1) and Shc compete for a limited pool of Grb2 in mediating insulin downstream signaling. J. Biol. Chem. 269, 31,107–31,114.
Pruett, W., Yuan, Y., Rose, E., Batzer, A. G., Harada, N., and Skolnik, E. Y. (1995) Association between GRB2/Sos and insulin receptor substrate 1 is not sufficient for activation of extracellular signal-regulated kinases by interleukin-4: implications for Ras activation by insulin. Mol. Cell. Biol. 15, 1778–1785.
Takada, T., Matozaki, T., Takeda, H., Fukunaga, K., Noguchi, T., Fujioka, Y., et al. (1998) Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J. Biol. Chem. 273, 9234–9242.
Fukunaga, K., Noguchi, T., Takeda, H., Matozaki, T., Hayashi, Y., Itoh, H., et al. (2000) Requirement for protein-tyrosine phosphatase SHP-2 in insulin-induced activation of c-Jun NH(2)-terminal kinase. J. Biol. Chem. 275, 5208–5213.
Kharitonenkov, A., Chen, Z., Sures, I., Wang, H., Schilling, J., and Ullrich, A. (1997) A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386, 181–186.
Hu, Q., Klippel, A., Muslin, A. J., Fantl, W. J., and Williams, L. J. (1995) Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268, 100–102.
Rodriguez-Viciana, P., Warne, P. H., Vanhaesebroeck, B., Waterfield, M. D., and Downward, J. (1996) Activation of phospho-inositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 15, 2442–2451.
Rodriguez-Viciana, P., Warne, P. H., Dhand, R., Vanhaesebroeck, B., Gout, I., Fry, M. J., et al. (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532.
Rubio, I., Rodriguez-Viciana, P., Downward, J., and Wetzker, R. (1997) Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem. J. 326, 891–895.
Asano, T., Kanda, A., Katagiri, H., Nawano, M., Ogihara, T., Inukai, K., et al. (2000) p110beta is up-regulated during differentiation of 3T3-L1 cells and contributes to the highly insulin-responsive glucose transport activity. J. Biol. Chem. 25, 17,671–17,676.
Katagiri, H., Asano, T., Ishihara, H., Inukai, K., Shibasaki, Y., Kikuchi, M., et al. (1996) Overexpression of catalytic subunit p110α of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. J. Biol. Chem. 271, 16,987–16,990.
Otsu, M., Hiles, I., Gout, I., Fry, M. J., Ruiz-Larrea, F., Panayotou, G., et al. (1991) Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle T/pp60c-src complexes, and PI3-kinase. Cell 65, 91–104.
Pons, S., Asano, T., Glasheen, E., Miralpeix, M., Zhang, Y. T., Fisher, T. L., et al. (1995) The structure and function of p55(PIK) reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol. Cell. Biol. 15, 4453–4465.
Antonetti, D. A., Algenstaedt, P., and Kahn, C. R. (1996) Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunit of phosphatidylinositol 3-kinase in muscle and brain. Mol. Cell. Biol. 16, 2195–2203.
Fruman, D. A., Cantley, L. C., and Carpenter, C. L. (1996) Structural organization and alternative splicing of the murine phosphoinositide 3-kinase p85 alpha gene. Genomics 37, 113–121.
Inukai, K., Anai, M., Van Breda, E., Hosaka, T., Katagiri, H., Funaki, M., et al. (1996) A novel 55-kDa regulatorysubunit for phosphatidylinositol 3-kinase structurally similar to p55PIK Is generated by alternative splicing of the p85alpha gene. J. Biol. Chem. 271, 5317–5320.
Alessi, D. R. and Downes, C. P. (1998) The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta 1436, 151–164.
Backer, J. M., Myers, M. G., Jr., Shoelson, S. E., Chin, D. J., Sun, X.-J., Miralpeix, M., et al. (1992) Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 11, 3469–3479.
Myers, M. G., Backer, J. M., Sun, X. J., Shoelson, S., Hu, P., Schlessinger, J., et al. (1992) IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc. Natl. Acad. Sci. USA 89, 10,350–10,354.
Shoelson, S. E., Sivaraja, M., Williams, K. P., Hu, P., Schlessinger, J., and Weiss, M. A. (1993) Specific phosphopeptide binding regulates a conformational change in the PI 3-kinase SH2 domain associated with enzyme activation. EMBO J. 12, 795–802.
Rordorf-Nikolic, T., VanHorn, D. J., Chen, D. X., White, M. F., and Backer, J. M. (1995) Regulation of phosphatidylinositol 3′-kinase bytyrosyl phosphoproteins—Full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. J. Biol. Chem. 270, 3662–3666.
Fruman, D. A., Rameh, L. E., and Cantley, L. C. (1999) Phosphoinositide binding domains: embracing 3-phosphate. Cell 97, 817–820.
Rameh, L. E. and Cantley, L. C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274, 8347–8350.
Venkateswarlu, K., Oatey, P. B., Tavare, J. M., and Cullen, P. J. (1998) Insulin-dependent translocation of ARNO to the plasma membrane of adipocytes requires phosphatidylinositol 3-kinase. Curr. Biol. 8, 463–466.
Oatey, P. B., Venkateswarlu, K., Williams, A. G., Fletcher, L. M., Foulstone, E. J., Cullen, P. J., et al. (1999) Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3-L1 adipocytes. Biochem. J. 344, 511–518.
Yang, C., Watson, R. T., Elmendorf, J. S., Sacks, D. B. and Pessin, J. E. (2000) Calmodulin antagonists inhibit insulin-stimulated GLUT4 (glucose transporter 4) translocation by preventing the formation of phosphatidylinositol 3,4,5-trisphosphate in 3T3L1 adipocytes. Mol. Endocrinol. 14, 317–326.
Le Good, J. A., Ziegler, W. H., Parekh, D. B., Alessi, D. R., Cohen, P., and Parker, P. J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045.
Toker, A. and Newton, A. C. (2000) Cellular signaling: pivoting around PDK-1. Cell 103, 185–188.
Wymann, M. P. and Pirola, L. (1998) Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta 1436, 127–150.
Duronio, V., Scheid, M. P. and Ettinger, S. (1998) Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell. Signal 10, 233–239.
Vanhaesebroeck, B. and Alessi, D. R. (2000) The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346, 561–576.
Cheatham, B., Vlahos, C. J., Cheatham, L., Wang, L., Blenis, J., and Kahn, C. R. (1994) Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis and glucose transporter translocation. Mol. Cell. Biol. 14, 4902–4911.
Sharma, P. M., Egawa, K., Huang, Y., Martin, J. L., Huvar, I., Boss, G. R., et al. (1998) Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273, 18,528–18,537.
Sakaue, H., Ogawa, W., Takata, M., Kuroda, S., Kotani, K., Matsumoto, M., et al. (1997) Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormoneor hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol. Endocrinol. 11, 1552–1562.
Vollenweider, P., Clodi, M., Martin, S. S., Imamura, T., Kavanaugh, W. M., and Olefsky, J. M. (1999) An SH2 domain-containing 5′ inositolphosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement. Mol. Cell. Biol. 19, 1081–1091.
Nakashima, N., Sharma, P. M., Imamura, T., Bookstein, R., and Olefsky, J. M. (2000) The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 275, 12,889–12,895.
Okada, T., Kawano, Y., Sakakibara, R., Hazeki, O., and Ui, M. (1994) Essential role of phophatidylinositol 3-kinase in insulin-induced glucose transport and antilypolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269, 3568–3573.
Martin, S. S., Haruta, T., Morris, A. J., Klippel, A., Williams, L. T., and Olefsky, J. M. (1996) Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 271, 17,605–17,608.
Frevert, E. U. and Kahn, B. B. (1997) Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol. Cell. Biol. 17, 190–198.
Frevert, E. U., Bjorbaek, C., Venable, C. L., Keller, S. R., and Kahn, B. B. (1998) Targeting of constitutively active phosphoinositide 3-kinase to GLUT4-containing vesicles in 3T3-L1 adipocytes. J. Biol. Chem. 273, 25,480–25,487.
Terauchi, Y., Tsuji, Y., Satoh, S., Minoura, H., Murakami, K., Okuno, A., et al. (1999) Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 21, 230–235.
Fruman, D. A., Snapper, S. B., Yballe, C. M., Davidson, L., Yu, J. Y., Alt, F. W., et al. (1999) Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science 283, 393–397.
Fruman, D. A., Mauvais-Jarvis, F., Pollard, D. A., Yballe, C. M., Brazil, D., et al. (2000) Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat. Genet. 26, 379–382.
Kohn, A. D., Summers, S. A., Birnbaum, M. J., and Roth, R. A. (1996) Expression of a constitutively active Akt ser/thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31,372–31,378.
Cong, L. N., Chen, H., Li, Y., Zhou, L., McGibbon, M. A., Taylor, S. I., et al. 1997 Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol. Endocrinol. 11, 1881–1890.
Hajduch, E., Alessi, D. R., Hemmings, B. A., and Hundal, H. S. (1998) Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47, 1006–1013.
Kohn, A. D., Barthel, A., Kovacina, K. S., Boge, A., Wallach, B., Summers, S. A., et al. (1998) Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J. Biol. Chem. 273, 11,937–11,943.
Wang, Q., Somwar, R., Bilan, P. J., Liu, Z., Jin, J., Woodgett, J. R., et al. (1999) Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 19, 4008–4018.
Heller-Harrison, R. A., Morin, M., Guilherme A., and Czech, M. P. (1996) Insulin-mediated targeting of phosphatidylinositol 3-kinase to GLUT4-containing vesicles. J. Biol. Chem. 271, 10,200–10,204.
Kupriyanova, T. A. and Kandror, K. V. (1999) Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J. Biol. Chem. 274, 1458–1464.
Calera, M. R., Martinez, C., Liu, H., Jack, A. K., Birnbaum, M. J., and Pilch, P. F. (1998) Insulin increases the association of Akt-2 with Glut4-containing vesicles. J. Biol. Chem. 273, 7201–7204.
Kitamura, T., Ogawa, W., Sakaue, H., Hino, Y., Kuroda, S., Takata, M., et al. (1998) Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol. Cell. Biol. 18, 3708–3717.
Kotani, K., Ogawa, W., Matsumoto, M., Kitamura, T., Sakaue, H., Hino, Y., et al. (1998) Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 18, 6971–6982.
Kotani, K., Ogawa, W., Hashiramoto, M., Onishi, T., Ohno, S., and Kasuga, M. (2000) Inhibition of insulin-induced glucose uptake by atypical protein kinase C isotype-specific interacting protein in 3T3-L1 adipocytes. J. Biol. Chem. 275, 26,390–26,395.
Standaert, M. L., Bandyopadhyay, G., Perez, L., Price, D., Galloway, L., Poklepovic, A., et al. (1999) Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 274, 25,308–25,316.
Bandyopadhyay, G., Standaert, M. L., Zhao, L., Yu, B., Avignon, A., Galloway, L., et al. (1997) Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J. Biol. Chem. 272, 2551–2558.
Bandyopadhyaya, G., Standaert, M. L., Kikkawa, U., Ono, Y., Moscat, J., and Farese, R. V. (1999) Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem. J. 337, 461–470.
Chen, D., Elmendorf, J. S., Olson, A. L., Li, X., Earp, H. S., and Pessin, J. E. (1997) Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J. Biol. Chem. 272, 27,401–27,410.
Kanzaki, M., Watson, R. T., Artemyev, N. O., and Pessin, J. E. (2000) The trimeric GTP-binding protein (G(q)/G(11)) alpha subunit is required for insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes. J. Biol. Chem. 275, 7167–7175.
Suzuki, Y., Shibata, H., Inoue, S., and Kojima, I. (1992) Stimulation of glucose transport by guanine nucleotides in permeabilized rat adipocytes. Biochem. Biophys. Res. Commun. 189, 572–580.
Elmendorf, J. S., Chen, D., and Pessin, J. E. (1998) Guanosine 5′-O-(3-thiotriphosphate) (GTPgammaS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J. Biol. Chem. 273, 13,289–13,296.
Baldini, G., Holman, R., Charron, M. J., and Lodish, H. F. (1991) Insulin and nonhydrolyzable GTP analogues induce translocation of GLUT4 to the plasma membrane in α-toxin-permeablilized rat adipose cells. J. Biol. Chem. 266, 4037–4040.
Imamura, T., Vollenweider, P., Egawa, K., Clodi, M., Ishibashi, K., Nakashima, N., et al. (1999) G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19, 6765–6774.
Imamura, T., Ishibashi, K., Dalle, S., Ugi, S., and Olefsky, J. M. (1999) Endothelin-1-induced GLUT4 translocation is mediated via G alpha (q/11) protein and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. J. Biol. Chem. 274, 33,691–33,695.
Yeh, J. I., Gulve, E. A., Rameh, L., and Birnbaum, M. J. (1995) The effects of wortmannin on rat skeletal muscle—dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J. Biol. Chem. 270, 2107–2111.
Lee, A. D., Hansen, P. A., and Holloszy, J. O. (1995) Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett. 361, 51–54.
Lund, S., Holman, G. D., Schmitz, O., and Pedersen, O. (1995) Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc. Natl. Acad. Sci. USA 92, 5817–5821.
Hayashi, T., Hirshman, M. F., Kurth, E. J., Winder, W. W., and Goodyear, L. J. (1998) Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47, 1369–1373.
Kurth-Kraczek, E. J., Hirshman, M. F., Goodyear, L. J., and Winder, W. W. (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48, 1667–1671.
Buhl, E. S., Jessen, N., Schmitz, O., Pedersen, S. B., Pedersen, O., Holman, G. D., et al. (2001) Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-d-ribofusanoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50, 12–17.
Kishi, K., Yuasa, T., Minami, A., Yamada, M., Hagi, A., Hayashi, H., et al. (2000) AMP-Activated protein kinase is activated by the stimulations of G(q)-coupled receptors. Biochem. Biophys. Res. Commun. 276, 16–22.
Krook, A., Whitehead, J. P., Dobson, S. P., Griffiths, M. R., Ouwens, M., Baker, C., et al. (1997) Two naturally occurring insulin receptor tyrosine kinase domain mutants provide evidence that phosphoinositide 3-kinase activation alone is not sufficient for the mediation of insulin's metabolic and mitogenic effects. J. Biol. Chem. 272, 30,208–30,214.
Isakoff, S. J., Taha, C., Rose, E., Marcusohn, J., Klip, A., and Skonik, E. Y. (1995) The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl. Acad. Sci. USA 92, 10,247–10,251.
Guilherme, A. and Czech, M. P. (1998) Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by betal-integrin signaling in rat adipocytes. J. Biol. Chem. 273, 33,119–33,122.
Jiang, T., Sweeney, G., Rudolf, M. T., Klip, A. Traynor-Kaplan, A., and Tsien, R. Y. (1998) Membrane-permeant esters of phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 11,017–11,024.
Ribon, V. and Saltiel, A. R. (1997) Insulin stimulates tyrosine phosphorylation of the protooncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem. J. 324, 839–845.
Sawasdikosol, S., Pratt, J. C., Meng, W., Eck, M. J., and Burakoff, S. J. (2000) Adapting to multiple personalities Cbl is also a RING finger ubiquitin ligase. Biochim. Biophys. Acta 1471, M1-M12.
Ribon, V., Herrera, R., Kay, B. K., and Saltiel, A. R. (1998) A role for CAP, a novel, multifunctional Src homology 3 domain-containing protein in formation of actin stress fibers and focal adhesions. J. Biol. Chem. 273, 4073–4080.
Ribon, V., Printen, J. A., Hoffman, N. G., Kay, B. K., and Saltiel, A. R. (1998) A novel, multifunctional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18, 872–879.
Ahmed, Z., Smith, B. J., and Pillay, T. S. (2000) The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475, 31–44.
Moodie, S. A., Alleman-Sposeto, J., and Gustafson, T. A. (1999) Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274, 11,186–11,193.
Galisteo, M. L., Dikic, I., Batzer, A. G., Langdon, W. Y., and Schlessinger, J. (1995) Tyrosine phosphorylation of the c-Cbl protooncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J. Biol. Chem. 270, 20,242–20,245.
Fukazawa, T., Miyake, S., Band, V., and Band, H. (1996) Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J. Biol. Chem. 271, 14,554–14,559.
Wang, Y., Yeung, Y. G., Langdon, W. Y., and Stanley, E. R. (1996) c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated and membrane-targeted following CSF-1 stimulation of macrophages. J. Biol. Chem. 271, 17–20.
Yokouchi, M., Kondo, T., Houghton, A., Bartkiewicz, M., Horne, W. C., Zhang, H., et al. (1999) Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J. Biol. Chem. 274, 31,707–31,712.
Joazeiro, C. A., Wing, S. S., Huang, H., Leverson, J. D., Hunter, T., and Liu, Y. C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312.
Baumann, C. A., Chokshi, N., Saltiel, A. R., and Ribon, V. (2000) Cloning and characterization of a functional peroxisome proliferator activator receptor-gamma-responsive element in the promoter of the CAP gene. J. Biol. Chem. 275, 9131–9135.
Ribon, V., Johnson, J. H., Camp, H. S., and Saltiel, A. R. (1998) Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc. Natl. Acad. Sci. USA 95, 14,751–14,756.
Baumann, C. A., Ribon, V., Kanzaki, M., Thurmond, D. C., Mora, S., Shigematsu, S., et al. (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207.
Nystrom, F. H., Chen, H., Cong, L. N., Li, Y., and Quon, M. J. (1999) Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Mol. Endocrinol. 13, 2013–2024.
Gustavsson, J., Parpal, S., Karlsson, M., Ramsing, C., Thorn, H., Borg, M., et al. (1999) Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13, 1961–1971.
Bickel, P. E., Scherer, P. E., Schnitzer, J. E., Oh, P., Lisanti, M. P., and Lodish, H. F. (1997) Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272, 13,793–13,802.
Volonte, D., Galbiati, F., Li, S., Nishiyama, K., Okamoto, T., and Lisanti, M. P. (1999) Flotillins/cavetellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 274, 12,702–12,709.
Simons, K. and Ikonen, E. (1997) Functional rafts in cell membranes. Nature 387, 569–572.
Kurzchalia, T. V. and Parton, R. G. (1999) Membrane microdomains and caveolae. Curr. Opin. Cell. Biol. 11, 424–431.
Anderson, R. G. (1993) Caveolae: where incoming and outgoing messengers meet. Proc. Natl. Acad. Sci. USA 90, 10,909–10,913.
Schlegel, A., Volonte, D., Engelman, J. A., Galbiati, F., Mehta, P., Zhang, X. L., et al. (1998) Crowded little caves: structure and function of caveolae. Cell Signal 10, 457–463.
Muller, G. and Frick, W. (1999) Signlling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell. Mol. Life Sci. 56, 945–970.
Stahlhut, M., Sandvig, K., and van Deurs, B. (2000) Caveolae: uniform structures with multiple functions in signaling, cell growth and cancer. Exp. Cell Res. 261, 111–118.
Shaul, P. W. and Anderson, R. G. (1998) Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 275, L843-L851
Okamoto, T., Schlegel, A., Scherer, P. E., and Lisanti, M. P. (1998) Caveolin, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273, 5419–5422.
Couet, J., Sargiacomo, M., and Lisanti, M. P. (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 272, 30,429–30,438.
Moffett, S., Brown, D. A., and Linder, M. E. (2000) Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275, 2191–2198.
Shaul, P. W., Smart, E. J., Robinson, L. J., German, Z., Yuhanna, I. S., Ying, Y., et al. (1996) Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 271, 6518–6522.
Shenoy-Scaria, A. M., Dietzen, D. J., Kwong, J., Link, D. C., and Lublin, D. M. (1994) Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J. Cell. Biol. 126, 353–363.
Li, S., Couet, J., and Lisanti, M. P. (1996) Src tyrosine kinases, Galpha subunits and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 271, 29,182–29,190.
Wary, K. K., Mariotti, A., Zurzolo, C., and Giancotti, F. G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625–634.
Song, K. S., Li, S., Okamoto, T., Quilliam, L. A., Sargiacom, O. M., and Lisanti, M. P. (1996) Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271, 9690–9697.
Ro, S., Luetterforst, R., Harding, A., Apolloni, A., Etheridge, M., Stang, E., et al. (1999) Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell. Biol. 1, 98–105.
Pike, L. J., and Casey, L. (1996) Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolinenriched membrane domains. J. Biol. Chem. 271, 26,453–26,456.
Pike, L. J. and Miller, J. M. (1998) Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem. 273, 22,298–22,304.
Prior, I. A., Harding, A., Yan, J., Sluimer, J., Parton, R. G., and Hancock, J. F. (2001) GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell. Biol. 3, 368–375.
Roy, S., Luetterforst, R., Harding, A., Apolloni, A., Etheridge, M., Stang, E., et al. (1999) Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell. Biol. 1, 98–105.
Hancock, J. F., Paterson, H., and Marshall, C. J. (1990) A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133–139.
Hancock, J. F., Magee, A. I., Childs, J. E., and Marshall, C. J. (1989) All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167–1177.
Scherer, P. E., Lisanti, M. P., Baldini, G., Sargiacomo, M., Mastick, C. C., and Lodish, H. F. (1994) Induction of caveolin during adipogenesis and association of GLUT4 with caveolinrich vesicles. J. Cell. Biol. 127, 1233–1243.
Song, K. S., Scherer, P. E., Tang, Z., Okamoto, T., Li, S., Chafel, M., et al. (1996) Expression of caveolin-3 in skeletal, cardiac and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271, 15,160–15,165.
Scherer, P. E., Lewis, R. Y., Volonte, D., Engelman, J. A., Galbiati, F., Couet, J., et al. (1997) Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 272, 29,337–29,346.
Smith, R. M., Harada, S., Smith, J. A., Zhang, S., and Jarett, L. (1998) Insulin-induced protein tyrosine phosphorylation cascade and signalling molecules are localized in a caveolin-enriched cell membrane domain. Cell Signal. 10, 355–362.
Mastick, C. C. and Saltiel, A. R. (1997) Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J. Biol. Chem. 272, 20,706–20,714.
Yamamoto, M., Toya, Y., Schwencke, C., Lisanti, M. P., Myers, M. G., and Ishikawa, Y. (1998) Caveolin is an activator of insulin receptor signaling. J. Biol. Chem. 273, 26,962–26,968.
Parpal, S., Karlsson, M., Thorn, H., and Stralfors, P. (2000) Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via IRS-1, but not for MAP-kinase control. J. Biol. Chem. 56, 11–12.
Chiang, S.-H., Baumann, C. A., Kanzaki, M., Watson, R. T., Thurmond, D. C., Macara, I. G., et al. (2001) Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of the small GTP binding protein TC10. Nature 410, 944–948.
Reedquist, K. A., Fukazawa, T., Panchamoorthy, G., Langdon, W. Y., Shoelson, S. E., Druker, B. J., et al. (1996) Stimulation through the T cell receptor induces Cbl association with Crk proteins and the guanine nucleotide exchange protein C3G. J. Biol. Chem. 271, 8435–8442.
Scaife, R. M. and Langdon, W. Y. (2000) c-Cbl localizes to actin lamellae and regulates lamellipodia formation and cell morphology. J. Cell. Sci. 113, (Pt. 2), 215–226.
Uemura, N. and Griffin, J. D. (1999) The adapter protein Crkl links Cbl to C3G after integrin ligation and enhances cell migration. J. Biol. Chem. 274, 37,525–37532.
Zhu, T., Goh, E. L., LeRoith, D., and Lobie, P. E. (1998) Growth hormone stimulates the formation of a multiprotein signaling complex involving p130(Cas) and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J. Biol. Chem. 273, 33,864–33,875.
Kyono, W. T., de Jong, R., Park, R. K., Liu, Y., Heisterkamp, N., Groffen, J., et al. (1998) Differential interaction of Crkl with Cbl or C3G, Hef-1, and gamma subunit immunoreceptor tyrosine-based activation motif in signaling of myeloid high affinity Fc receptor for IgG (Fc gamma RI). J. Immunol. 161, 5555–5563.
Ishiki, M., Sasaoka, T., Ishihara, H., Imamura, T., Usui, I., Takata, Y., et al. (1997) Evidence for functional roles of Crk-II in insulin and epidermal growth factor signaling in Rat-1 fibroblasts overexpressing insulin receptors. Endocrinology 138, 4950–4958.
Neudauer, C. L., Joberty, G., Tatsis, N., and Macara, I. G. (1998) Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr. Biol. 8, 1151–1160.
Imagawa, M., Tsuchiya, T., and Nishihara, T. (1999) Identification of inducible genes at the early stage of adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 254, 299–305.
Watson, R. T., Shigematsu, S., Chiang, S.-H., Mora, S., Kanzaki, M., Macara, I. G., Saltiel, A. R., and Pessin, J. E. (2001) Lipid raft microdomain compartmentalization of TC10 is required for insulin signaling and GLUT4 translocation. J. Cell Biol. 154, 829–840.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanzaki, M., Pessin, J.E. Signal integration and the specificity of insulin action. Cell Biochem Biophys 35, 191–209 (2001). https://doi.org/10.1385/CBB:35:2:191
Issue Date:
DOI: https://doi.org/10.1385/CBB:35:2:191