Abstract
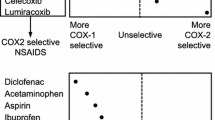
Celecoxib, a nonsteroidal anti-inflammatory drug (NSAID), is the first specific inhibitor of cyclo-oxygenase-2 (COX-2) approved to treat patients with rheumatism and osteoarthritis. Preliminary data suggest that celecoxib also has analgesic and anticancer properties. The selective inhibition of COX-2 is thought to lead to a reduction in the unwanted effects of NSAIDs. Upper gastrointestinal complication rates in clinical trials are significantly lower for celecoxib than for traditional nonselective NSAIDs (e.g. naproxen, ibuprofen and diclofenac).
The rate of absorption of celexocib is moderate when given orally (peak plasma drug concentration occurs after 2 to 4 hours), although the extent of absorption is not known. Celexocib is extensively protein bound, primarily to plasma albumin, and has an apparent volume of distribution of 455 ± 166L in humans. The area under the plasma concentration-time curve (AUC) of celecoxib increases in proportion to increasing oral doses between 100 and 800mg. Celecoxib is eliminated following biotransformation to carboxylic acid and glucuronide metabolites that are excreted in urine and faeces, with little drug (2%) being eliminated unchanged in the urine. Celecoxib is metabolised primarily by the cytochrome P450 (CYP) 2C9 isoenzyme and has an elimination half-life of about 11 hours in healthy individuals. Racial differences in drug disposition and pharmacokinetic changes in the elderly have been reported for celecoxib.
Plasma concentrations (AUC) of celecoxib appear to be 43% lower in patients with chronic renal insufficiency [glomerular filtration rate 2.1 to 3.6 L/h (35 to 60 ml/min)] compared with individuals with healthy renal function, with a 47% increase in apparent clearance. Compared with healthy controls, it has been reported that the steady-state AUC is increased by approximately 40% and 180% in patients with mild and moderate hepatic impairment, respectively.
Celecoxib does not appear to interact with warfarin, ketoconazole or methotrexate; however, clinically significant drug interactions with fluconazole and lithium have been documented. As celecoxib is metabolised by CYP2C9, increased clinical vigilance is required during the coadministration of other substrates or inhibitors of this enzyme.







Similar content being viewed by others
References
Simon LS, Lanza FL, Lipsky PE, et al. Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase-2 inhibitor. Arthritis Rheum 1998; 41: 1591–602.
Hubbard RC, Koepp RJ, Yu S, et al. SC-58635 (celecoxib), a novel COX-2 selective inhibitor, is effective as a treatment for osteoarthritis (OA) in a short-term pilot study [abstract 1188]. Arthritis Rheum 1996; 39 Suppl.: S226.
Hubbard RC, Mehlisch DR, Jasper DR, et al. SC-58635, a highly selective inhibitor of COX-2, is an effective analgesic in an acute post-surgical pain model [abstract]. J Invest Med 1996; 44: 293A.
Zhang Y, Shaffer A, Portanova J, et al. Inhibition of cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharmacol Exp Ther 1997; 283: 1069–75.
Penning TD, Talley JJ, Bertenshaw SR, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-trifluoromethyl- 1H-pyrazol- l-yl]benzene sulfonamide (SC-58635, celecoxib). J Med Chem 1997; 40: 1347–65.
Khanna IK, Weier JJ, Yu Y, et al. 1,2-Diarylimidazoles as potent, cyclooxygenase-2 selective, and orally active antiinflammatory agents. J Med Chem 1997; 40: 1634–47.
Khanna IK, Weier JJ, Yu Y, et al. 1,2-Diarylimidazoles as potent, and selective inhibitors of cyclooxygenase-2. J Med Chem 1997; 40: 1619–33.
Patterson R, Bello AE, Lefkowith J. Immunologic tolerability profile of celecoxib. Clin Ther 1999 Dec; 21: 2065–79.
Food and Drug Administration. New drug application #20998: clinical pharmacology/biopharmaceutics review section celecoxib. Bethesda (MD): FDA, 1998.
Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 1998; 38: 97–120.
Cryer B, Dubois A. The advent of highly selective inhibitors of cyclooxygenase: a review. Prost Other Lipid Mediat 1998; 56: 341–61.
Mitchell JA, Evans TW. Cyclooxygenase-2 as a therapeutic target. Inflamm Res 1998; 47 Suppl. 2: S88–92.
Bolten WW. Scientific rationale for specific inhibition of COX-2. J Rheumatol 1998; 51 Suppl.: 2–7.
McAdam BF, Lawson-Catela F, Mardini IS, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 1999; 96: 272–7.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993; 10: 1093–5.
Karim A, Tolbert D, Piergies A, et al. Celecoxib, a specific COX-2 inhibitor, lacks significant drug-drug interactions with methotrexate or warfarin [abstract 1698]. Arthritis Rheum 1998; 41(9 Suppl.): S315.
Karim A, Tolbert D, Piergies A, et al. Celecoxib biotransformation: fluconazole but not ketoconazole substantially increases celecoxib exposure in man [abstract 4022]. Pharm Res 1998; 1 Suppl. 1: S626.
Karim A, Tolbert D, Burton E, et al. SC-58635 (celecoxib): a highly selective inhibitor of cyclooxygenase-2 disposition kinetics in man and identification of its major CYP450 isozyme in its biotransformation [abstract 3469]. Pharm Res 1997; 14 Suppl. 11: S617.
Hamilton LC, Mitchell JA, Tomlinson AM, et al. Synergy between cyclo-oxygenase-2 induction and arachidonic acid supply in vivo: consequences for nonsteroidal antiinflammatory drug efficacy. FASEB J 1999; 13: 245–51.
Day RO, Francis H, Vial J, et al. Naproxen concentrations in plasma and synovial fluid and effects on prostanoid concentrations. J Rheumatol 1995; 22: 2295–303.
Henry DA. Side-effects of non-steroidal anti-inflammatory drugs. Baillieres Clin Rheumatol 1988; 2: 425–54.
Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions: a review of the literature. Drugs 1998; 56: 783–99.
Wang SL, Huang J, Lai MD, et al. Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics 1995; 5: 37–42.
Miners JP, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Cin Pharmacol 1998; 45: 525–38.
Kalow W. Pharmacogenetics in biological perspective. Pharmacol Rev 1997; 49: 369–79.
Vane JR, Mitchell JA, Appleton I, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthetase in inflammation. Proc Natl Acad Sci USA 1994; 91: 2046–50.
Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1993; 268: 6610–4.
Mahmud T, Rafi SS, Scott DL, et al. Nonsteroidal antiinflammatory drugs and uncoupling of mitochondrial oxidative phosphorylation. Arthritis Rheum 1996; 39: 1998–2003.
Lagenbach R, Morham SG, Tiano HF, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995; 83: 483–92.
Morham SG, Langenbach RL, Loftin CD, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 1995; 83: 473–82.
Dinchuk JE, Car BD, Focht RJ, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 1995; 378: 406–9.
Schror K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 1997; 23: 349–56.
Patrono C. Cyclooxygenase enzymes in human vascular disease. In: Vane J, editor. Selective COX-2 inhibitors: pharmacology, clinical effects and therapeutic potential. London: Kluwer Academic Publishers, 1997.
Baker CS, Hall RJ, Evans TJ, et al. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol 1999; 19(3): 646–55.
Mehlisch DR, Hubbard RC, Isakson P, et al. Analgesic efficacy and plasma levels of a highly selective inhibitor of COX-2 (SC-58635) in patients with post-surgical dental pain [abstract PIII-2]. Clin Pharmacol Ther 1997; 61: 195.
Riendeau D, Charleson S, Cromlish W, et al. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal antiinflammatory drugs (NSATDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol 1997; 75: 1088–95.
Gaw-Mengle L, Hubbard RC, Karim A, et al. A study of the platelet effects of SC-58635, a novel COX-2 selective inhibitor [abstract 374]. Arthritis Rheum 1997; 40 Suppl. 9: S93.
Leese PT, Hubbard RC, Karim A, et al. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000; 40(2): 124–32.
Day RO, Furst DE, Droomgoole SH, et al. Relationship of serum naproxen concentration to efficacy in rheumatoid arthritis. Clin Pharm Ther 1982; 31: 733–40.
Anderson GD, Hauser SD, Mcgarity KL, et al. Selective inhibition of cyclooxygenase-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest 1996; 97: 2672–9.
Walker JS, Sheather-Reid RB, Carmody JJ, et al. Nonsteroidal antiinflammatory drugs in rheumatoid arthritis and osteoarthritis: support for the concept of ‘responders’ and ‘non-responders’. Arthritis Rheum 1997; 40: 1944–54.
Zhao SZ, Hatoum HT, Hubbard AC, et al. Effect of celecoxib, a novel COX-2 inhibitor, on health-related quality of life patients with osteoarthritis of the knee [abstract 348]. Arthritis Rheum 1997; 40 suppl. 9: S88.
Hubbard RC, Geis GS, Woods E, et al. Efficacy, tolerability, and safety of celecoxib, a specific COX-2 inhibitor, in osteoarthritis [abstract 982]. Arthritis Rheum 1998; 41(9 Suppl.): S196.
Geis GS, Stead H, Morant S, et al. Efficacy and safety of celecoxib, a specific COX-2 inhibitor, in patients with rheumatoid arthritis [abstract 1990]. Arthritis Rheum 1998; 41(9 Suppl.): S364.
Lanza Fl, Rack MF, Callison DA, et al. Apilot endoscopic study of the gastroduodenal effects of SC-58635, a novel COX-2 selective inhibitor [abstract A]. Gastroenterology 1997; 112(4): 194.
Mohammed S, Croom II DW Gastropathy due to celecoxib, a cyclooxygenase-2 inhibitor. N Engl J Med 1999; 340: 2005–6.
Reuben SS, Steinberg R. Gastric perforation associated with the use of celecoxib. Anesthesiology 1999; 91: 1548–9.
Less GI Disorders with celecoxib than conventional NSAIDs. Inpharma 1998 Dec 12; 1167: 22.
Khan KN, Venturini CM, Bunch RT, et al. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol Pathol 1998; 5: 612–20.
Rossat J, Maillard M, Nussberger J, et al. Renal effects of selective cyclooxygenase-2 inhibition in normotensive salt-depleted subjects. Clin Pharmacol Ther 1999; 66: 76–84.
Leach M, Hamilton LC, Olbrich A, et al. Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br J Pharmacol 1998; 124: 586–92.
Rosenberg L, Palmer JR, Zauber AG, et al. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large bowel cancer. J Natl Cancer Inst 1996; 83: 355–8.
Eberhardt CE, Coffey RJ, Radhika A, et al. Up regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1996; 107: 1183–8.
Kargman SL, O’Neill GP, Vikers PJ, et al. Expression of prostaglandin G/H synthase-1 and -2 protein in human colorectal cancer. Cancer Res 1991; 55: 2556–9.
Ryseck RP, Raynoschek C, Macdonald-Bravo H, et al. Identification of an immediate early gene, PGHS B, whose protein product has prostaglandin synthase/cyclooxygenase activity. Cell Gr Diff 1993; 3: 443–50.
Jones DA, Carlton DP, McIntyre TM, et al. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 1993; 268: 9049–54.
Kawamori T, Rao CV, Seibert K, et al. Chemopreventative activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 1998; 58: 409–12.
Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventative properties in colon carcinogenesis. Cancer Res 1996; 56: 4566–9.
Masferrer J, Leahy K, Kobolt C, et al. Celecoxib: a specific COX-2 inhibitor with anti-inflammatory, analgesic and anticancer activities [abstract]. Naunyn-Schmiedeberg’s Arch Pharmacol 1998; 358: 8.
Fischer SM, Lo HH, Gordon GB, et al. Chemopreventative activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinogen 1999; 25: 231–40.
Watkins DN, Lenzo JC, Segal A, et al. Expression and localization of cyclo-oxygenase isoforms in non-small cell lung cancer. Eur Respir J 1999; 14: 412–8.
Kitamura Y, Shimohama S, Koike H, et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease brains. Biochem Biophys Res Commun 1999; 254: 582–6.
McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol 1998; 33: 371–8.
Guerguerian AM, Hardy P, Bhattacharya M, et al. Expression of cyclooxygenases in ductus arteriosus of fetal and newborn pigs. Am J Obstet Gynecol 1998; 179(6 Pt 1): 1618–26.
Karim A, Tolbert D, Hunt TL, et al. Celecoxib, a specific COX-2 inhibitor, has no significant effect on methotrexate pharmacokinetics in patients with rheumatoid arthritis. J Rheumatol 1999 Dec; 26(12): 2539–43.
Karim A, Piergies A, Tolbert D, et al. Selective inhibitor of cyclooxygenase-2 (COX-2) SC-58635 (celecoxib), does not affect kinetics or dynamics of warfarin [abstract 606]. J Clin Pharmacol 1998; 38: 880.
Acknowledgements
Professor Ric Day is on the Australian advisory board for celecoxib.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davies, N.M., McLachlan, A.J., Day, R.O. et al. Clinical Pharmacokinetics and Pharmacodynamics of Celecoxib. Clin Pharmacokinet 38, 225–242 (2000). https://doi.org/10.2165/00003088-200038030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200038030-00003