Abstract
Warfarin is one of the most widely prescribed oral anticoagulants. However, optimal use of the drug has been hampered by its >10-fold interpatient variability in the doses required to attain therapeutic responses. Pharmacogenetic polymorphism of cytochrome P450 (CYP) may be associated with impaired elimination of warfarin and exaggerated anticoagulatory responses to the drug in certain patients.
Clinically available warfarin is a racemic mixture of (R)- and (S)-warfarin, and the (S)-enantiomer has 3 to 5 times greater anticoagulation potency than its optical congener. Both enantiomers are eliminated extensively via hepatic metabolism with low clearance relative to hepatic blood flow. CYP2C9 is almost exclusively responsible for the metabolism of the pharmacologically more active (S)-enantiomer.
Several human allelic variants of CYP2C9 have been cloned, designated as CYP2C9*1 (reference sequence or wild-type allele), CYP2C9*2, CYP2C9*3and CYP2C9*4, respectively. The allelic frequencies for these variants differ considerably among different ethnic populations. Caucasians appear to carry the CYP 2C9*2 (8 to 20%) and CYP2C9*3 (6 to 10%) variants more frequently than do Asians (0% and 2 to 5%, respectively).
The metabolic activities of the CYP2C9 variants have been investigated in vitro. The catalytic activity of CYP2C9*3 expressed from cDNA was significantly less than that of CYP2C9*1. Human liver microsomes obtained from individuals heterozygous for CYP2C9*3 showed significantly reduced (S)-warfarin 7-hydroxylation as compared with those obtained from individuals genotyped as CYP2C9*1.
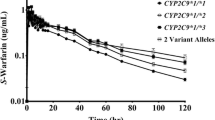
The influence of the CYP2C9*3 allele on the in vivo pharmacokinetics of (S)-warfarin has been studied in Japanese patients. Patients with the homozygous CYP2C9*3 genotype, as well as those with the heterozygous CYP2C9*1/*3 genotype, had significantly reduced clearance of (S)-warfarin (by 90 and 60%, respectively) compared with those with homozygous CYP2C9*1.
The maintenance dosages of warfarin required in Japanese patients with heterozygous and homozygous CYP2C9*3 mutations were significantly lower than those in patients with CYP2C9*1/*1. In addition, 86% of British patients exhibiting adequate therapeutic responses with lower maintenance dosages of warfarin (<1.5 mg/day) had either the CYP2C9*2 or CYP2C9*3 mutation singly or in combination, whereas only 38% of randomly selected patients receiving warfarin carried the corresponding mutations. Furthermore, the former group showed more frequent episodes of major bleeding associated with warfarin therapy.
These data indicate that the CYP2C9*3 allele may be associated with retarded elimination of (S)-warfarin and the resulting clinical effects. However, relationships between CYP2C9 genotype, enzyme activity, metabolism of probe substrates, dosage requirements and bleeding complications should be interpreted with caution, and further studies are required.




Similar content being viewed by others
References
Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 1998; 114: 445S–69S
O’Reilly RA. Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther 1974; 16: 348–54
Breckenridge A, Orme M, Wesseling H, et al. Pharmacokinetics and pharmacodynamics of the enantiomers of warfarin in man. Clin Pharmacol Ther 1974; 15: 424–30
Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol 1992; 5: 54–9
Kaminsky LS, Zhang Z-Y. Human P450 metabolism of warfarin. Pharmacol Ther 1997; 73: 67–74
Goldstein JA, de Morais SMF. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 1994; 4: 285–99
Wilting J, van der Giesen WF, Janssen LHM, et al. The effect of albumin conformation on the binding of warfarin to human serum albumin: the dependence of the binding of warfarin to human serum albumin on the hydrogen, calcium, and chloride ion concentrations as studied by circular dichroism, fluorescence and equilibrium dialysis. J Biol Chem 1980; 255: 3032–7
Porter RS, Sawyer WT. Warfarin. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. Vancouver (WA): Applied Therapeutics Inc, 1992: 31 (1)-(46)
Wilkinson GR, Shand DG. A physiological approach to hepatic drug clearance. Clin Pharmacol Ther 1975; 18: 377–90
Takahashi H, Kashima T, Kimura S, et al. Determination of unbound warfarin enantiomers in human plasma and 7- hydroxywarfarin in human urine by chiral stationary-phase liquid chromatography with ultraviolet or fluorescence and on-line circular dichroism detection. J Chromatogr B 1997; 701:71–80
Toon S, Low LK, Gibaldi M, et al. The warfarin-sulfinpyrazone interactions: stereochemical considerations. Clin Pharmacol Ther 1986; 39: 15–24
Chan E, McLachlan A, O’Reilly R, et al. Stereochemical as pects of warfarin drug interactions: use of a combined pharmacokinetic-pharmacodynamic model. Clin Pharmacol Ther 1994; 56: 286–94
Takahashi H, Sato T, Shimoyama Y, et al. Potentiation of anti coagulant effect of warfarin caused by enantioselective metabolic inhibition by the uricosuric agent benzbromarone. Clin Pharmacol Ther 1999; 66: 569–81
Takahashi H, Kashima T, Kimura S, et al. Pharmacokinetic in teraction between warfarin and a uricosuric agent, bucolome: application of in vitro approaches to predicting in vivo reduction of (S)-warfarin clearance. Drug Metab Dispos 1999; 27: 1179–86
Kunze KL, Trager WF. Warfarin-fluconazole interaction III: a rational approach to management of a metabolically based drug interaction. Drug Metab Dispos 1996; 24: 429–35
Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. BrJ Clin Pharmacol 1998; 45: 525–38
Toon S, Hopkins KJ, Garstang FM, et al. Enoxacin-warfarin interaction: pharmacokinetic and stereochemical aspects. Clin Pharmacol Ther 1987; 42: 33–41
Niopas I, Toon S, Aarons L, et al. The effect of cimetidine on the steady-state pharmacokinetics and pharmacodynamics of warfarin in humans. Eur J Clin Pharmacol 1999; 55: 399–404
Unge P, Svedberg LE, Nordgren A, et al. A study of the inter action of omeprazole and warfarin in anticoagulated patients. Br J Clin Pharmacol 1992; 34: 509–12
Gidal BE, Sorkness CA, McGill KA, et al. Evaluation of a potential enantioselective interaction between ticlopidine and warfarin in chronically anticoagulated patients. Ther Drug Monit 1995; 17: 33–8
Abernethy DR, Kaminsky LS, Dickinson TH. Selective inhibition of warfarin metabolism by diltiazem in humans. J Pharmacol Exp Ther 1991; 257; 411–5
Shimada T, Yamazaki H, Miura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994; 270: 414–23
Bourrie M, Meunier V, Berger Y, et al. Role of cytochrome P-4502C9 in irbesartan oxidation by human liver microsomes. Drug Metab Dispos 1999; 27: 288–96
Gill HJ, Tjia JF, Kitteringham NR, et al. The effect of genetic polymorphisms in CYP2C9 on sulphamethoxazole N-hydr-oxylation. Pharmacogenetics 1999; 9: 43–53
Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics 1999; 9: 71–80
Oscarson M, Ingelman-Sunberg M, Daly AK, et al. Human cytochrome P450 (CYP) genes. Available from: URL: http://www.imm.ki.se/CYPalleles/ [Accessed 2001 July 10]
Schwarz UI, Choo EF, Dresser GK, et al. Identification of anew CYP2C9 variant in African-Americans. Clin Pharmacol Ther 2000; 67: 169
Imai J, Ieiri I, Mamiya K, et al. Polymorphism of the cytochrome P450 (CYP) 2C9 gene in Japanese epileptic patients: genetic analysis of the CYP2C9 locus. Pharmacogenetics 2000; 10: 85–9
Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 1996; 6: 341–9
London SJ, Daly AK, Leathart JBS, et al. Lung cancer risk in relation to the CYP2C9*1/CYP2C9*2 genetic polymorphism among African-American and Caucasians in Los Angeles county, California. Pharmacogenetics 1996; 6: 527–33
Stubbins MJ, Harries LW, Smith G, et al. Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics 1996; 6: 429–39
Furuya H, Fernandez-Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995; 5: 389–92
Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood 2000; 96: 1816–9
Gaedigk A, Leeder JS, Sellers EM, et al. Cytochrome P450 CYP2C9allele frequencies in Canadian native Indians (CNI). Clin Pharmacol Ther 2000; 67: 168
Brockmöller J, Rost KL, Gross D, et al. Phenotyping of CYP2C19 with enantiospecific HPLC-quantification of R- and 5-mephenytoin and comparison with the intron 4/exon 5 G→A splice site mutation. Pharmacogenetics 1995; 5: 80–8
Cascorbi I, Ackermann E, Sachse C, et al. A novel CYP2C9 intron 2 T/C transition and linkage to mutations Leu359 and Cys 144. Clin Pharmacol Ther 1998; 65: 198
Yasar Ü, Eliasson E, Dahl ML, et al. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun 1999; 254: 628–31
Aynacioglu AS, Brockmöller J, Bauer S, et al. Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 1999; 48: 409–15
Freeman BD, Zehnbauer BA, McGrath S, et al. Cytochrome P450 polymorphisms are associated with reduced warfarin dose. Surgery 2000; 128: 281–5
Wang SL, Huang JD, Lai MD, et al. Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics 1995; 5: 37–42
Nasu K, Kubota T, Ishizaki T. Genetic analysis of CYP2C9poly-morphism in a Japanese population. Pharmacogenetics 1997; 7: 405–9
Takahashi H, Kashima T, Nomizo Y, et al. Metabolism of warfarin enantiomers in Japanese patients with heart disease having different CYP2C9 and CYP2C19 genotypes. Clin Pharmacol Ther 1998; 63: 519–28
Kimura M, Ieiri I, Mamiya K, et al. Genetic polymorphism of cytochrome P450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monit 1998; 20: 243–7
Nebert DW. Suggestions for the nomenclature of human alleles: relevance to ecogenetics, pharmacogenetics and molecular epidemiology. Pharmacogenetics 2000; 10: 279–90
Shintani M, Ieiri I, Inoue K, et al. Genetic polymorphism and functional characterization in 5′-flanking region of human cytochrome P450 (CYP) 2C9 gene: in vitro and in vivo studies. Xenobio Metab Dispos 2000; 15; S307
Haining RL, Hunter AP, Veronese ME, et al. Allelic variants of human cytochrome P450 2C9: baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity, and prochiral selectivity of the wild-type and I359Lmutant forms. Arch Biochem Biophys 1996; 333: 447–58
Rettie AE, Wienkers LC, Gonzalez FJ, et al. Impaired (S)-war-farin metabolism catalyzed by the R144C allelic variant of CYP2C9. Pharmacogenetics 1994; 4: 39–42
Takahashi H, Kashima T, Nomoto S, et al. Comparisons be tween in-vitro and in-vivo metabolism of (5)-warfarin: catalytic activities of cDNA-expressed CYP2C9, its Leu359 variant and their mixture versus unbound clearance in patients with the corresponding CYP2C9 genotypes. Pharmacogenetics 1998; 8: 365–73
Yamazaki H, Inoue K, Chiba K, et al. Comparative studies on the catalytic roles of cytochrome P450 2C9 and its Cys- and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol 1998; 56: 243–51
Takahashi K, Tainaka H, Kobayashi K, et al. CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with seven substrates. Pharmacogenetics 2000; 10: 95–104
Crespi CL, Miller VP. The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH: cytochrome P450 oxidoreductase. Pharmacogenetics 1997; 7: 203–10
Rettie AE, Haining RL, Bajpai M, et al. A common genetic basis for idiosyncratic toxicity of warfarin and phenytoin. Epilepsy Res 1999; 35: 253–5
Tracy TS, Rosenbluth BW, Wrighton SA, et al. Role of cytochrome P450 2C9 and an allelic variant in the 4′-hydroxyla- tion of (R)- and (S)-flurbiprofen. Biochem Pharmacol 1995; 49; 1269–75
Chang TKH, Yu L, Goldstein JA, et al. Identification of the polymorphically expressed CYP2C19 and the wild-type CYP2C9-Ile359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics 1997; 7: 211–21
Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem 1992; 267: 83–90
Ieiri I, Tainaka H, Morita T, et al. Catalytic activity of three variants (Ile, Leu, and Thr) at amino acid residue 359 in human CYP2C9 gene and simultaneous detection using singlestrand conformation polymorphism analysis. Ther Drug Monit 2000; 22: 237–44
Odani A, Hashimoto Y, Otsuki Y, et al. Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics ofphenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther 1997; 62: 287–92
Mamiya K, Ieiri I, Shimamoto J, et al. The effects of genetic polymorphisms of CYP2C9 and CYP2C19 on phenytoin metabolism in Japanese adult patients with epilepsy: studies in stereoselective hydroxylation and population pharmacokinetics. Epilepsia 1998; 39: 1317–23
Inoue K, Yamazaki H, Imiya K, et al. Relationship between CYP2C9 and 2C19 genotypes and tolbutamide methyl hydroxylation and S-mephenytoin 4′-hydroxylation activities in livers of Japanese and Caucasian populations. Pharmacogenetics 1997; 7: 103–13
Bhasker CR, Miners JO, Coulter S, et al. Allelic and functional variability of cytochrome P4502C9. Pharmacogenetics 1997; 7: 51–8
Steward DJ, Haining RL, Henne KR, et al. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics 1997; 7: 361–7
Aithal GP, Day CP, Kesteven PJL, et al. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 1999; 353: 717–9
Aithal GP, Day CP, Kesteven PJL, et al. Warfarin dose requirement and CYP2C9 polymorphisms — Authors’ reply. Lancet 1999; 353: 1972–3
Margaglione M, Colaizzo D, D’Andrea G, et al. Genetic modulation of oral anticoagulation with warfarin. Thromb Haemost 2000; 84: 775–8
McCrea JB, Cribb A, Rushmore T, et al. Phenotypic and genotypic investigations of a healthy volunteer deficient in the conversion of losartan to its active metabolite E-3174. Clin Pharmacol Ther 1999; 65: 348–52
Shimamoto J, Ieiri I, Urae A, et al. Lack of differences in diclofenac (a substrate for CYP2C9) pharmacokinetics in healthy volunteers with respect to the single CYP2C9*3 allele. Eur J Clin Pharmacol 2000; 56: 65–8
Stearns RA, Chakravarty PK, Chen R, et al. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Drug Metab Dispos 1995; 23: 207–15
Chan E, McLachlan AJ, Pegg M, et al. Disposition of warfarin enantiomers and metabolites in patients during multiple dosing with rac-warfarin. Br J Clin Pharmacol 1994; 37: 563–9
Dickinson RG, Hooper WD, Patterson M, et al. Extent of urinary excretion of p-hydroxyphenytoin in healthy subjects given phenytoin. Ther Drug Monit 1985; 7: 283–9
Brogden RN, Heel RC, Pakes GE, et al. Glipizide: a review of its pharmacological properties and therapeutic uses. Drugs 1979; 18: 329–53
Veronese ME, Miners JO, Rees DLP, et al. Tolbutamide hydroxylation in humans: lack of bimodality in 106 healthy subjects. Pharmacogenetics 1993; 3: 86–93
Benet LZ, Oie S, Schwartz JB. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill, 1996: 1707–92
Acknowledgements
The authors thank Dr Ichiro Ieiri, Tottori University, for his helpful discussion during the preparation of the article. This paper was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (12670703) and Japanese Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, H., Echizen, H. Pharmacogenetics of Warfarin Elimination and its Clinical Implications. Clin Pharmacokinet 40, 587–603 (2001). https://doi.org/10.2165/00003088-200140080-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140080-00003