Abstract
Although the past few years have seen an exponential growth of compounds of potential interest for the treatment of functional gastrointestinal (GI) tract disorders, the gap that still exists between basic and clinical research is easily noticed if one considers the relative paucity of drugs that have received marketing authorisation for the treatment of irritable bowel syndrome (IBS). Traditional efficacy outcomes in drug development for IBS include the ability of the compound to affect GI tract motility (i.e. to exert a prokinetic or an antispasmodic effect), which is thought to be of importance if a motor disorder is the underlying pathophysiological mechanism. More recently, altered visceral sensitivity to a distending stimulus has been suggested to be a key pathophysiological feature, at least in some patients, and has become a target for therapeutic interventions. However, there is now growing consensus that the primary outcome measure in the treatment of functional disorders are those that reflect overall control of the patient’s symptoms (pain, diarrhoea, constipation) in everyday situations such as the clinical global improvement scales. Although, in general, guidelines on the design of treatment trials for functional GI tract disorders advise against subcategorisation of patients according to the main symptom (because of symptom instability), subcategorisation indeed makes sense especially in IBS (constipation- or diarrhoea-predominant). Compounds with a specific indication for each subpopulation of patients are now emerging.
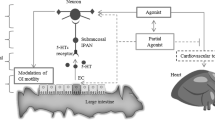
The rationale for investigations on serotonin (5-hydroxytryptamine; 5-HT) receptor ligands in IBS rests mainly on the fact that serotonin, which may be released by enterochromaffin-like cells in the GI tract as well as from other sources, has a number of well documented motor effects on the GI tract and can produce hyperalgesia in several experimental models. Serotonin receptors belonging to the 5-HT3 and 5-HT4 subtype are the most extensively studied in gastroenterology, although hitherto ‘orphan’ receptor subtypes, such as the 5-HT7 and the 5-HT1b/d receptors, are now emerging.
Among 5-HT3 receptor antagonists, alosetron was recently approved for the treatment of diarrhoea-predominant IBS and is an example of a compound that, at least theoretically, may act at multiple levels: by inhibiting visceral sensitivity, by increasing compliance, and by inhibiting excitatory 5-HT3 receptors located on both ascending and descending neuronal pathways involved in peristalsis. For this reason, 5-HT3 receptor antagonists may slow transit, hence the specific indication of alosetron in diarrhoea-predominant IBS. However, alosetron has been recently withdrawn by the manufacturer because of safety concerns.
Hypomotility remains an attractive therapeutic target in IBS and the new generation of prokinetics includes several partial agonists at the 5-HT4 receptor, such as tegaserod (HTF-919) and prucalopride (R0-93877). In addition, preliminary evidence suggests that 5-HT4 receptors may also be involved in the modulation of visceral sensitivity. Second-generation 5-HT4 receptor agonists seem to be devoid of the QT-prolonging effects observed in some clinical circumstances with cisapride and may be more active at the colonic level. Piboserod (SB-207266A) is a 5-HT4 receptor antagonist under development for the treatment of diarrhoea-predominant IBS.
Finally, interest in 5-HT7 and 5-HT1b/d receptor subtypes stems from the observation that the former receptors mediate smooth muscle relaxation (at least in the human colon), whereas sumatriptan (a 5-HT1B/Dreceptor agonist) can affect GI tract motility and visceral sensitivity.








Similar content being viewed by others
References
Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology 1997; 112: 2120–37
Anonymous. American Gastroenterological Association medical position statement: irritable bowel syndrome. Gastroenterology 1997; 112: 2118–9
Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut 1999; 45 Suppl. 2: II1–II5
Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut 1999; 45 Suppl. 2: II43–II47
Serra J, Azpiroz F, Malagelada JR. Intestinal gas dynamics and tolerance in humans. Gastroenterology 1998; 115: 542–50
Tonini M. Recent advances in the pharmacology of gastrointestinal prokinetics. Pharmacol Res 1996; 33: 217–26
Klein KB. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology 1988; 95: 232–41
Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994; 107: 271–93
De Ponti F, Malagelada JR. Functional gut disorders: from motility to sensitivity disorders: a review of current and investigational drugs for their management. Pharmacol Ther 1998; 80: 49–88
Sanger GJ. Hypersensitivity and hyperreactivity in the irritable bowel syndrome: an opportunity for drug discovery. Dig Dis 1999; 17: 90–9
Pandolfino N, Howden C, Kahrilas P. Motility-modifying agents and management of disorders of gastrointetsinal motility. Gastroenterology 2000; 118: S32–S47
Bueno L, Fioramonti J, Garcia-Villar R. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. III. Visceral afferent pathways: a source of new therapeutic targets for abdominal pain. Am J Physiol 2000; 278: G670–G676
Coulie B, Szarka LA, Camilleri M, et al. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 2000; 119: 41–50
Whitehead WE, Delvaux M, and the Working Team. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. Dig Dis Sci 1997; 42: 223–41
Delvaux M, Louvel D, Mamet JP, et al. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 1998; 12: 849–55
Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999; 44: 400–6
Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 1996; 347: 150–3
Bueno L, Fioramonti J, Delvaux M, et al. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology 1997; 112: 1714–43
Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 1996; 111: 1683–99
Farthing MJ. Irritable bowel syndrome: new pharmaceutical approaches to treatment. Baillieres Best Pract Res Clin Gastroenterol 1999; 13: 461–71
Mertz H, Fass R, Kodner A, et al. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol 1998; 93: 160–5
Peghini PL, Katz PO, Castell DO. Imipramine decreases oesophageal pain perception in human male volunteers. Gut 1998; 42: 807–13
Gorelick AB, Koshy SS, Hooper FG, et al. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol 1998; 275: G460–G6
Malagelada JR. Review article: clinical pharmacology models of irritable bowel syndrome. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 57–64
Camilleri M. Ten secrets for development of drugs for functional gastrointestinal diseases. Gastroenterology 2000; 118: 653
Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, et al. Design of treatment trials for functional gastrointestinal disorders. Gut 1999; 45 Suppl. 2: II69–II77
Klein KB. Assessment of treatment outcome in the functional gastrointestinal disorders. In: Corazziari E, editor. Approach to the patient with chronic gastrointestinal disorders. Milan: Messaggi, 1999: 545–56
Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 77–82: 77–82
Gershon MD. Review article: roles played by 5-hydroxytrypt-amine in the physiology of the bowel. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 15–30
Read NW, Gwee KA. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther 1994; 62: 159–73
Briejer MR, Akkermans LM, Schuurkes JA. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol Rev 1995; 47: 631–51
Prins NH, Briejer MR, Van Bergen PJ, et al. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol 1999; 128: 849–52
Galligan JJ. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurons. In: Gaginella TS, Galligan JJ, editors. Serotonin and gastrointestinal function. Boca Raton (FL): CRC Press, 1995: 109–26
Tonini M, De Ponti F. Serotonin modulation of gastrointestinal motility. In: Gaginella TS, Galligan JJ, editors. Serotonin and gastrointestinal function. Boca Raton (FL): CRC Press, 1995: 53–84
Galligan JJ, Surprenant A, Tonini M, et al. Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am J Physiol 1988; 255: G603–G611
Dietrich C, Kilbinger H. 5-HT1A receptor-mediated inhibition of acetylcholine release from guinea pig myenteric plexus: potential mechanisms. Neuropharmacology 1996; 35: 483–8
Kuemmerle JF, Murthy KS, Grider JR, et al. Coexpression of 5-HT2A and 5-HT4 receptors coupled to distinct signaling pathways in human intestinal muscle cells. Gastroenterology 1995; 109: 1791–800
Coulie B, Tack J, Sifrim D, et al. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol 1999; 276: G373–G7
Coulie B, Tack J, Maes B, et al. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am J Physiol 1997; 272: G902–G8
Borman RA, Burleigh DE. 5-HT1D and 5-HT2B receptors mediate contraction of smooth muscle in human small intestine. Ann NY Acad Sci 1997; 812: 222–3
Tack J, Coulie B, Wilmer A, et al. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut 2000; 46: 468–73
Borman RA, Burleigh DE. Evidence for the involvement of a 5-HT4 receptor in the secretory response of human small intestine to 5-HT. Br J Pharmacol 1993; 110: 927–8
Stoner MC, Arcuni JC, Lee J, et al. A selective 5-HT4 receptor agonist induces cAMP-mediated Cl− efflux from rat colonocytes [abstract]. Gastroenterology 1999; 116: A648
Humphrey PP, Bountra C, Clayton N, et al. Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 31–8
Miyata K, Kamato T, Nishida A, et al. Pharmacologic profile of (R)-5-[(l-methyl-3-indolyl) carbonyl]-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride (YM060), a potent and selective 5-hydroxytryptamine3 receptor antagonist, and its enantiomer in the isolated tissue. J Pharmacol Exp Ther 1991; 259: 15–21
Messori E, Candura SM, Coccini T, et al. 5-HT3 receptor involvement in descending reflex relaxation in the rabbit isolated distal colon. Eur J Pharmacol 1995; 286: 205–8
Miyata K, Kamato T, Nishida A, et al. Role of serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther 1992; 261: 297–303
Kadowaki M, Nagakura Y, Tomoi M, et al. Effect of FK1052, a potent 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptor dual antagonist, on colonic function in vivo. J Pharmacol Exp Ther 1993; 266: 74–80
Sakurai-Yamashita Y, Yamashita K, Yoshimura M, et al. Differential localization of 5-hydroxytryptamine3 and 5-hydroxy-tryptamine4 receptors in the human rectum. Life Sci 2000; 66: 31–4
Sakurai-Yamashita Y, Yamashita K, Kaibara M, et al. Differential distribution of 5-hydroxytryptamine3 receptor in the colon between human and guinea pig. Chin J Physiol 1999; 42: 195–8
Tuladhar BR, Kaisar M, Naylor RJ. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br J Pharmacol 1997; 122: 1174–8
Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (5HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 1999; 288: 93–7
Talley NJ, Phillips SF, Miller LJ, et al. Effect of selective 5HT3 antagonist (GR 38032F) on small intestinal transit and release of gastrointestinal peptides. Dig Dis Sci 1989; 34: 1511–5
Steadman CJ, Talley NJ, Phillips SF, et al. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc 1992; 67: 732–8
Talley NJ, Phillips SF, Haddad A, et al. GR 38032F (ondansetron), a selective 5-HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci 1990; 35: 477–80
Gore S, Gilmore IT, Haigh CG, et al. Colonie transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther 1990; 4: 139–44
von der Ohe M, Hanson RB, Camilleri M. Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut 1994; 35: 536–41
Maxton DG, Morris J, Whorwelll PJ. Selective 5-hydroxytrypt-amine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther 1996; 10: 595–9
Prior A, Read NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther 1993; 7: 175–80
von der Ohe MR, Camilleri M, Kvols LK. A 5-HT3 antagonist corrects the postprandial colonic hypertonic response in car-cinoid diarrhea. Gastroenterology 1994; 106: 1184–9
Sanger GJ. 5-Hydroxytryptamine and functional bowel disorders. Neurogastroenterol Motil 1996; 8: 319–31
Moss HE, Sanger GJ. The effects of granisetron, ICS 205–930 and ondansetron on the visceral pain reflex induced by duodenal distension. Br J Pharmacol 1990; 100: 497–501
Miura M, Lawson DC, Clary EM, et al. Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci 1999; 44: 20–4
Banner SE, Sanger GJ. Differences between 5-HT3 receptor antagonists in modulation of visceral hypersensitivity. Br J Pharmacol 1995; 114: 558–62
Goldberg PA, Kamm MA, Setti-Carraro P, et al. Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron). Digestion 1996; 57: 478–83
Feinle C, Read NW. Ondansetron reduces nausea induced by gastroduodenal stimulation without changing gastric motility. Am J Physiol 1996; 261: G591–G597
Foster JM, Houghton LA, Whorwell PJ. Alosetron slows colonic transit in patients with irritable bowel syndrome (IBS) [abstract]. Gastroenterology 1997; 112: A732
Gunput MD, Sohail S, Frith L, et al. Alosetron, a 5-HT3 receptor antagonist, has no effect on oro-cecal transit time in man [abstract]. Dig Dis Sci 1996; 41: 1896
Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther 2000; 14: 775–82
Schuurkes JAJ, Meulemans AL, Obertop H, et al. 5-HT4 receptors on the human stomach [abstract]. J Gastrointest Motil 1991; 3: 199
Sakurai-Yamashita Y, Takada K, Takemura K, et al. Ability of mosapride to bind to 5-HT4 receptor in the human stomach. Jpn J Pharmacol 1999; 79: 493–6
Burleigh DE, Trout SJ. Evidence against an acetylcholine releasing action of cisapride in the human colon. Br J Clin Pharmacol 1985; 20: 475–8
Burke TA, Sanger GJ. Regionally selective cholinergic stimulation by BRL 24924 in the human isolated gut. Br J Clin Pharmacol 1988; 26: 261–5
Briejer MR, Akkermans LM, Meulemans AL, et al. Cisapride and a structural analogue, R 76,186, are 5-hydroxytryptamine4 (5-HT4) receptor agonists on the guinea-pig colon ascendens. Naunyn Schmiedebergs Arch Pharmacol 1993; 347: 464–70
Wardle KA, Sanger GJ. The guinea-pig distal colon-a sensitive preparation for the investigation of 5-HT4 receptor-mediated contractions. Br J Pharmacol 1993; 110: 1593–9
Tam FS, Hillier K, Bunce KT, et al. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br J Pharmacol 1995; 115: 172–6
McLean PG, Coupar IM. Stimulation of cyclic AMP formation in the circular smooth muscle of human colon by activation of 5-HT4-like receptors. Br J Pharmacol 1996; 117: 238–9
Prins NH, Akkermans LMA, Lefebvre RA, et al. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol 2000; 131: 927–32
Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiate peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol 1996; 270: 778–82
Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea-pig intestine. Gastroenterology 1996; 111: 1281–90
Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998; 115: 370–80
Gunput MD. Review article: clinical pharmacology of alosetron. Aliment Pharmacol Ther 1999; 13 Suppl. 2: 70–6
Balfour JA, Goa KL, Perry CM. Alosetron [see guest commentaries]. Drugs 2000; 59: 511–8
Kozlowski CM, Green A, Grundy D, et al. The 5-HT3 receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut 2000; 46: 474–80
Zerbib F, Bruleyd, V, Oriola RC, et al. Alosetron does not affect the visceral perception of gastric distension in healthy subjects. Aliment Pharmacol Ther 1994; 8: 403–7
Thumshirn M, Coulie B, Camilleri M, et al. Effects of alosetron on gastrointestinal transit time and rectal sensation in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2000; 14: 869–78
Clayton NM, Sargent R, Butler A, et al. The pharmacological properties of the novel selective 5-HT3 receptor antagonist, alosetron, and its effects on normal and perturbed small intestinal transit in the fasted rat. Neurogastroenterol Motil 1999; 11: 207–17
Bouras EP, Camilleri M, Burton DD, et al. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut 1999; 44: 682–6
Scott LJ, Perry CM. Tegaserod. Drugs 1999; 58: 491–6
Zhou H, Khalilieh S, Lau H, et al. Effect of meal timing not critical for the pharmacokinetics of tegaserod (HTF 919). J Clin Pharmacol 1999; 39: 911–9
Mannaert E, Van Bortel I, Woestenborghs R, et al. Single-dose and once-daily pharmacokinetics and effects of food on prucalopride in humans [abstract]. Gut 1999; 45 Suppl. 5: A137
Appel S, Kumle A, Hubert M, et al. First pharmacokinetic-pharmacodynamic study in humans with a selective 5-hydroxytryptamine4 receptor agonist. J Clin Pharmacol 1997; 37: 229–37
Appel-Dingemanse S, Lemarechal MO, Kumle A, et al. Integrated modelling of the clinical pharmacokinetics of SDZ HTF 919, a novel selective 5-HT4 receptor agonist, following oral and intravenous administration. Br J Clin Pharmacol 1999; 47: 483–91
Bardhan KD, Bodemar G, Geldof H, et al. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2000; 14: 23–34
Jones RH, Holtmann G, Rodrigo L, et al. Alosetron relieves pain and improves bowel function compared with mebeverine in female nonconstipated irritable bowel syndrome patients. Aliment Pharmacol Ther 1999; 13: 1419–27
Camilleri M, Northcutt AR, Kong S, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 2000; 355: 1035–40
Pillans PI, Wood SM. Cisapride increases micturition frequency. J Clin Gastroenterol 1994; 19: 336–8
Tonini M, Messori E, Franceschetti GP, et al. Characterization of the 5-HT receptor potentiating neuromuscular cholinergic transmission in strips of human isolated detrusor muscle. Br J Pharmacol 1994; 113: 1–2
Puisieux FL, Adamantidis MM, Dumotier BM, et al. Cisapride-induced prolongation of cardiac action potential and early afterdepolarizations in rabbit Purkinje fibres. Br J Pharmacol 1996; 117: 1377–9
Tonini M, De Ponti F, Di Nucci A, et al. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther 1999; 13: 1585–91
Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol 2000; 36: 1–12
Napolitano C, Schwartz PJ, Brown AM, et al. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol 2000; 11: 691–6
De Ponti F, Poluzzi E, Montanaro N. QT interval prolongation by non-cardiac drugs: lessons to be learned by recent experience. Eur J Clin Pharmacol 2000; 56: 1–18
Michalets EL, Williams CR. Drug interactions with cisapride: clinical implications. Clin Pharmacokinet 2000; 39: 49–75
Carlsson L, Amos GJ, Andersson B, et al. Electrophysiological characterization of the prokinetic agents cisapride and mosapride in vivo and in vitro: implications for proarrhythmic potential? J Pharmacol Exp Ther 1997; 282: 220–7
Crema F, Modini C, Croci T, et al. Intestinal prokinesia by two esters of 4-amino-5-chloro-2-methoxybenzoic acid: involvement of 5-hydroxytryptamine-4 receptors and dissociation from cardiac effects in vivo. J Pharmacol Exp Ther 1999; 288: 1045–52
Drici MD, Ebert SN, Wang WX, et al. Comparison of tegaserod (HTF 919) and its main human metabolite with cisapride and erythromycin on cardiac repolarization in the isolated rabbit heart. J Cardiovasc Pharmacol 1999; 34: 82–8
Whorwell PJ, Krumholz S, Muller-Lissner S, et al. Tegaserod has a favorable safety and tolerability profile in patients with constipation predominant and alternating forms of irritable bowel syndrome (IBS) [abstract]. Gastroenterology 2000; 118 Suppl. 2: A1204
Nguyen A, Camilleri M, Kost LJ, et al. SDZHTF 919 stimulates canine colonic motility and transit in vivo. J Pharmacol Exp Ther 1997; 280: 1270–6
Emmanueal AV, Nicholls T, Roy AJ, et al. Prucalopride (PRU) improves colonic transit and stool frequency in patients (pts) with slow and normal transit constipation [abstract]. Gastroenterology 2000; 118 Suppl. 2: A846
Camilleri M, McKinzie S, Burton D, et al. Prucalopride accelerates small bowel and colonic transit in patients with chronic functional constipation (FC) or constipation-predominant irritable bowel syndrome (C-IBS) [abstract]. Gastroenterology 2000; 118 Suppl. 2: A845
Yoshida N, Omoya H, Kato S, et al. Pharmacological effects of the new gastroprokinetic agent mosapride citrate and its metabolites in experimental animals. Arzneimittelforschung 1993; 43: 1078–3
Mine Y, Yoshikawa T, Oku S, et al. Comparison of effect of mosapride citrate and existing 5-HTin4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther 1997; 283: 1000–8
Prather CM, Camilleri M, Zinsmeister AR, et al. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology 2000; 118: 463–8
Lefkowitz MP, Rueegg PC, Shi Y, et al. Validation of a global relief measure in two clinical trials of irritable bowel syndrome with tegaserod [abstract]. Gastroenterology 2000; 118 Suppl. 2: A145
Mueller-Lissner S, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor partial agonist, relieves key symptoms of irritable bowel syndrome (IBS) [abstract]. Gastroenterology 2000; 118 Suppl. 2: A175
Coelho AM, Rovira P, Fioramonti J, et al. Antinociceptive properties of HTF 919 (tegaserod), a 5-HT4 receptor partial agonist, on colorectal distension in rats [abstract]. Gastroenterology 2000; 118 Suppl. 2: A835
Poen AC, Felt-Bersma RJ, Van Dongen PA, et al. Effect of prucalopride, a new enterokinetic agent, on gastrointestinal transit and anorectal function in healthy volunteers. Aliment Pharmacol Ther 1999; 13: 1493–7
Emmanuel AV, Kamm MA, Roy AJ, et al. Effect of a novel prokinetic drug, R093877, on gastrointestinal transit in healthy volunteers. Gut 1998; 42: 511–6
Otten M, Schneider H, Wurzer H, et al. A double-blind, placebo controlled evaluation of safety and efficacy of 12-week, twice-daily treatment with prucaloprid in patients with chronic constipation. Gastroenterology 1999; 116: 1055
Anonymous. Mosapride price-listed in Japan. Scrip 1998; 2374: 26
Ruth M, Hamelin B, Röhss K, et al. The effect of mosapride, anovel prokinetic, on acid reflux variables in patients with gastro-oesohageal reflux disease. Aliment Pharmacol Ther 1998; 12: 35–40
Sanger GJ, Banner SE, Smith MI, et al. SB-207266: 5-HT4 receptor antagonism in human isolated gut and prevention of 5-HT-evoked sensitization of peristalsis and increased defaecation in animal models. Neurogastroenterol Motil 1998; 10: 271–9
Bharucha AE, Camilleri M, Haydock S, et al. Effects of a serotonin 5-HT4 receptor antagonist SB-207266 on gastrointetsinal motor and sensory function in humans. Gut 2000; 47: 667–74
Sanger GJ, Yoshida M, Yahyah M, et al. Increased defecation during stress or after 5-hydroxytryptophan: selective inhibition by the 5-HT4 receptor antagonist, SB-207266. Br J Pharmacol 2000; 130: 706–12
Cooper SM, Haydock SF, Tompson DJ, et al. A pharmacodynamic model of 5-HT4 receptor activation in man: antagonism by the 5-HT4 receptor antagonist SB-207266 [abstract]. Gastroenterology 1999; 116: A598
Houghton LA, Jackson NA, Whorwell PJ, et al. 5-HT4 receptor antagonism in irritable bowel syndrome: effect of SB-207266-A on rectal sensitivity and small bowel transit. Aliment Pharmacol Ther 1999; 13: 1437–44
Smith MI, Banner SE, Sanger GJ. 5-HT4 receptor antagonism potentiates inhibition of intestinal allodynia by 5-HT3 receptor antagonism in conscious rats. Neurosci Lett 1999; 271: 61–4
Houghton LA, Fowler P, Keene ON, et al. Effect of sumatriptan, a new selective 5HT1-like agonist, on liquid gastric emptying in man. Aliment Pharmacol Ther 1992; 6: 685–91
Tack J, Piessevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998; 115: 1346–52
Coulie B, Tack J, Gevers A, et al. Influence of sumatriptan-induced colonic relaxation on the perception of colonic distention in man [abstract]. Gastroenterology 1997; 112: A715
Coulie B, Tack J, Janssen J. Influence of buspirone-induced fundus relaxation on the perception of gastric distention in man [abstract]. Gastroenterology 1997; 112: A715
De Ponti F, Crema F, Nardelli G, et al. Blockade by the selective 5-HT1b/d receptor antagonist GR127935 of the effect of sumatriptan on canine gastric compliance [abstract]. Neurogastroenterol Motil 2000; 12: 383
Croci T, Landi M, Bianchetti A, et al. Drug-induced defaecation in rats: role of central 5-HT1a receptors. Br J Pharmacol 1995; 115: 203–9
Vanhoenacker P, Haegeman G, Leysen JE. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci 2000; 21: 70–7
Bueno L. New and future drugs in nerve-gut dysfunction. Ital J Gastroenterol Hepatol 1999; 31: 794–801
Toulouse M, Coelho AM, Fioramonti J, et al. Role of tachykinin NK2 receptors in normal and altered rectal sensitivity in rats. Br J Pharmacol 2000; 129: 193–9
Onori L, Aggio A, Taddei G, et al. Contribution of NK(2) tachykinin receptors to propulsion in the rabbit distal colon. Am J Physiol Gastrointest Liver Physiol 2000; 278: G137–G47
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Ponti, F., Tonini, M. Irritable Bowel Syndrome. Drugs 61, 317–332 (2001). https://doi.org/10.2165/00003495-200161030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161030-00001