Abstract
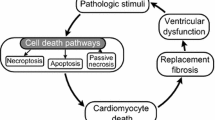
A large volume of experimental data supports the presence of apoptosis in failing hearts. Apoptosis in many types of cells results from exposure to cytotoxic cytokines or damaging agents. Cytotoxic cytokines such as tumor necrosis factor (TNF)-α; or Fas ligand (FasL) bind to their receptors to activate caspase-8, while damaging agents can cause mitochondrial release of cytochrome c, which can initiate activation of caspase-9. Caspase-8 or -9 can activate a cascade of caspases. The p53 protein is often required for damaging agent-induced apoptosis. An imbalance of proapoptotic factors versus prosurvival factors in the bcl-2 family precedes the activation of caspases. Given these typical changes of apoptosis found in many cell types, the apoptotic pathway in cardiomyocytes is somewhat unconventional since in vivo experimental data reveal that apoptosis does not appear to be controlled by TNF-α;, FasL, p53 or decrease of bcl-2. In vitro and in vivo studies suggest the importance of mitochondria and activation of caspases in cell death occurring in failing hearts. Oxidants, excessive nitric oxide, angiotensin II and catecholamines have been shown to trigger apoptotic death of cardiomyocytes. Eliminating these inducers reduces apoptosis and reverses the loss of contractile function in many cases, indicating the feasibility of the pharmacological application of antioxidants, nitric oxide synthetase inhibitors, ACE inhibitors, angiotensin II receptor antagonists and adrenergic receptor antagonists. Most inducers of apoptosis initiate a cascade of signaling events, including activation of the p38 mitogen-activated protein kinase. Small molecule inhibitors of p38 have been shown to be capable of preventing apoptosis and loss of contractile function associated with ischemia and reperfusion. Although further experimental work is needed, several studies have already indicated the beneficial effect of caspase inhibitors against cell loss and features of heart failure in vitro and in vivo. These studies indicate the importance of inhibiting apoptosis in therapeutic interventions against heart failure.



Similar content being viewed by others
References
Colucci WS, Braunwald E. In: Braunwald E, editor. Heart disease: a textbook of cardiovascular medicine. Pathophysiology of heart failure. Vol. 1. 5th ed. Phildelphia (PA): WB Saunders Company, 1997: 394–420
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26(4): 239–57
Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol 1995; 146(1): 3–15
Hale AJ, Smith CA, Sutherland LC, et al. Apoptosis: molecular regulation of cell death. Eur J Biochem 1996; 236(1): 1–26
Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 2000; 45(3): 528–37
Levin S, Bucci TJ, Cohen SM, et al. The nomenclature of cell death: recommendations of an ad hoc Committee of the Society of Toxicologic Pathologists. Toxicol Pathol 1999; 27(4): 484–90
Hongo M, Ryoke T, Ross J. Animal models of heart failure: recent developments and perspectives. Trends Cardiovasc Med 1997; 7(5): 161–7
Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res 1998; 39(1): 60–76
Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 1998; 39(1): 89–105
Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med 1997; 336(16): 1131–41
Narula J, Haider N, Virmani R, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 1996; 335(16): 1182–9
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267(5203): 1456–62
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998; 281(5381): 1305–8
Li H, Zhu H, Xu CJ, et al. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94(4): 491–501
Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281(5381): 1312–6
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999; 68: 383–424
Budihardjo I, Oliver H, Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15: 269–90
Barinaga M. Cell suicide: by ICE, not fire. Science 1994; 263(5148): 754–6
Peitsch MC, Mannherz HG, Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol 1994; 4: 37–41
Bortner CD, Olderburg NBE, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol 1995; 5: 21–6
Enari M, Sakahira H, Yokoyama H, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998; 391(6662): 43–50
Sakahira H, Enari M and Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998; 391(6662): 96–9
Walker PR, Sikorska M. New aspects of the mechanism of DNA fragmentation in apoptosis. Biochem Cell Biol 1997; 75(4): 287–99
Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281(5381): 1309–12
Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1998; 1366(1-2): 139–49
Higuchi M, Proske RJ, Yeh ET. Inhibition of mitochondrial respiratory chain complex I by TNF results in cytochrome c release, membrane permeability transition, and apoptosis. Oncogene 1998; 17(19): 2515–24
Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol 1998; 16: 395–419
Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science 1997; 277(5324): 370–2
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281(5381): 1322–6
Kluck RM, Bossy-Wetzel E, Green DR, et al. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997; 275(5303): 1132–6
Sheikh MS, Fornace AJ. Role of p53 family members in apoptosis. J Cell Physiol 2000; 182(2): 171–81
Polyak K, Xia Y, Zweier JL, et al. A model for p53-induced apoptosis. Nature 1997; 389(6648): 300–5
Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria: a potential role in apoptotic signaling. J Biol Chem 2000; 275(21): 16202–12
Schuler M, Bossy-Wetzel E, Goldstein JC, et al. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem 2000; 275(10): 7337–42
Evan G, Littlewood T. A matter of life and cell death. Science 1998; 281(5381): 1317–22
Frenkel J, Sherman D, Fein A, Accentuated apoptosis in normally developing p53 knockout mouse embryos following genotoxic stress. Oncogene 1999; 18(18): 2901–7
Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 1998; 67: 481–507
Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 2000; 275(50): 39435–43
Hetman M, Kanning K, Cavanaugh JE, et al. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 1999; 274(32): 22569–80
Wang Y, Huang S, Sah VP, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem 1998; 273(4): 2161–8
Hreniuk D, Garay M, Gaarde W, et al. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol Pharmacol 2001; 59(4): 867–74
Assefa Z, Vantieghem A, Garmyn M, et al. p38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J Biol Chem 2000; 275(28): 21416–21
Kennedy SG, Kandel ES, Cross TK, et al. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 1999; 19(8): 5800–10
Erhardt P, Schremser EJ, Cooper GM, et al. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol 1999; 19(8): 5308–15
Kharbanda S, Saxena S, Yoshida K, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem 2000; 275(1): 322–7
Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148(7): 2207–16
van Engeland M, Nieland LJ, Ramaekers FC, et al. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998; 31(1): 1–9
van Heerde WL, Robert-Offerman S, Dumont E, et al. Markers of apoptosis in cardiovascular tissues: focus on Annexin V Cardiovasc Res 2000; 45(3): 549–59
Elsasser A, Suzuki K, Schaper J. Unresolved issues regarding the role of apoptosis in the pathogenesis of ischemic injury and heart failure. J Mol Cell Cardiol 2000; 32(5): 711–24
Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res 2000; 86(11): 1107–13
Haunstetter A, Izumo S. Future perspectives and potential implications of cardiac myocyte apoptosis. Cardiovasc Res 2000; 45(3): 795–801
Cook SA, Poole-Wilson PA. Cardiac myocyte apoptosis. Eur Heart J 1999; 20(22): 1619–29
Williams RS. Apoptosis and heart failure. N Engl J Med 1999; 341(10): 759–60
Reed JC, Paternostro G. Postmitochondrial regulation of apoptosis during heart failure. Proc Natl Acad Sci USA 1999; 96(14): 7614–6
Diez J, Fortuno MA, Ravassa S. Apoptosis in hypertensive heart disease. Curr Opin Cardiol 1998; 13(5): 317–25
James TN. Apoptosis in cardiac disease. Am J Med 1999; 107(6): 606–20
Sabbah HN. Apoptotic cell death in heart failure. Cardiovasc Res 2000; 45(3): 704–12
Feuerstein G, Ruffolo RRJ, Yue T-L. Apoptosis and congestive heart failure. Trends Cardiovasc Med 1997; 7(7): 249–55
Yaoita H, Ogawa K, Maehara K. Apoptosis in relevant clinical situations: contribution of apoptosis in myocardial infarction. Cardiovasc Res 2000; 45(3): 630–41
Ohno M, Takemura G, Ohno A, et al. ’Apoptotic’ myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with In situ nick end-labeling. Circulation 1998; 98(14): 1422–30
Bachetti T, Ferrari R. The dynamic balance between heart function and immune activation. Eur Heart J 1998; 19(5): 681–2
Yamamoto A, Wenthold RJ, Zhang J, et al. Immunofluorescence techniques for the identification of immune effector cells in rat heart: applications to the study of the myocarditis induced by interleukin-2. J Mol Cell Cardiol 1995; 27(1): 307–19
Huber SA. T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res 2000; 45(3): 579–87
Patella V, Marino I, Arbustini E, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 1998; 97(10): 971–8
Engels W, Reiters PH, Daemen MJ, et al. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol 1995; 177(4): 423–9
Hara M, Matsumori A, Ono K, et al. Mast cells cause apoptosis of cardiomyocytes and proliferation of other intramyocardial cells in vitro. Circulation 1999; 100(13): 1443–9
Francis GS. TNF-alpha and heart failure: the difference between proof of principle and hypothesis testing. Circulation 1999; 99(25): 3213–4
Seta Y, Shan K, Bozkurt B, et al. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail 1996; 2(3): 243–9
Nishigaki K, Minatoguchi S, Seishima M, et al. Plasma Fas ligand, an inducer of apoptosis, and plasma soluble Fas, an inhibitor of apoptosis, in patients with chronic congestive heart failure. J Am Coll Cardiol 1997; 29(6): 1214–20
Toyozaki T, Hiroe M, Tanaka M, et al. Levels of soluble Fas ligand in myocarditis. Am J Cardiol 1998; 82(2): 246–8
Ing DJ, Zang J, Dzau VJ, et al. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res 1999; 84(1): 21–33
Yamaoka M, Yamaguchi S, Suzuki T, et al. Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol 2000; 32(6): 881–9
Yamamura T, Nakamura H, Yamamoto T, et al. Fas expression and apoptosis correlate with cardiac dysfunction in patients with dilated cardiomyopathy. Jpn Circ J 1999; 63(3): 149–54
Filippatos G, Leche C, Sunga R, et al. Expression of FAS adjacent to fibrotic foci in the failing human heart is not associated with increased apoptosis. Am J Physiol 1999; 277(2 Pt 2): H445–51
Kurrelmeyer KM, Michael LH, Baumgarten G, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci USA 2000; 97(10): 5456–61
Nelson DP, Setser E, Hall DG, et al. Proinflammatory consequences of transgenic fas ligand expression in the heart. J Clin Invest 2000; 105(9): 1199–208
Goossens V, De Vos K, Vercammen D, et al. Redox regulation of TNF signaling. Biofactors 1999; 10(2-3): 145–56
Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, et al. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol 1997; 273(1 Pt 1): G7–17
Long X, Boluyt MO, Hipolito ML, et al. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest 1997; 99(11): 2635–43
Xie Z, Koyama T, Abe K, et al. Upregulation of P53 protein in rat heart subjected to a transient occlusion of the coronary artery followed by reperfusion. Jpn J Physiol 2000; 50(1): 159–62
Leri A, Liu Y, Malhotra A, et al. Pacing-induced heart failure in dogs enhances the expression of p53 and p53-dependent genes in ventricular myocytes. Circulation 1998; 97(2): 194–203
Petrovic D, Zorc-Pleskovic R, Zorc M. Apoptosis and proliferation of cardiomyocytes in heart failure of different etiologies. Cardiovasc Pathol 2000; 9(3): 149–52
Chen QM, Bartholomew JC, Campisi J, et al. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 1998; 332(1): 43–50
Chen QM, Liu J, Merrett J. Apoptosis or senescence-like growth arrest: influence of cell cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J 2000; 347: 543–51
Kirshenbaum LA. Regulators of apoptosis in the heart: a matter of life and death. Can J Cardiol 1998; 14(3): 457–60
Webster KA, Discher DJ, Kaiser S, et al. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest 1999; 104(3): 239–52
Bialik S, Geenen DL, Sasson IE, et al. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest 1997; 100(6): 1363–72
Francis GS, Anwar F, Bank AJ, et al. Apoptosis, Bcl-2, and proliferating cell nuclear antigen in the failing human heart: observations made after implantation of left ventricular assist device. J Card Fail 1999; 5(4): 308–15
Saraste A, Pulkki K, Kallajoki M, et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 1999; 29(5): 380–6
Latif N, Khan MA, Birks E, et al. Upregulation of the Bcl-2 family of proteins in end stage heart failure. J Am Coll Cardiol 2000; 35(7): 1769–77
Ikeda S, Hamada M, Hiwada K. Contribution of non-cardiomyocyte apoptosis to cardiac remodelling that occurs in the transition from compensated hypertrophy to heart failure in spontaneously hypertensive rats. Clin Sci 1999; 97(2): 239–46
Condorelli G, Morisco C, Stassi G, et al. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 1999; 99(23): 3071–8
Narula J, Pandey P, Arbustini E, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 1999; 96(14): 8144–9
de Moissac D, Gurevich RM, Zheng H, et al. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol 2000; 32(1): 53–63
Koseki T, Inohara N, Chen S, et al. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A 1998; 95(9): 5156–60
Ekhterae D, Lin Z, Lundberg MS, et al. ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res 1999; 85(12): e70–7
Black SC, Huang JQ, Rezaiefar P, et al. Co-localization of the cysteine protease caspase-3 with apoptotic myocytes after in vivo myocardial ischemia and reperfusion in the rat. J Mol Cell Cardiol 1998; 30(4): 733–42
Holly TA, Drincic A, Byun Y, et al. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol 1999; 31(9): 1709–15
Jiang L, Huang Y, Yuasa T, et al. Elevated DNase activity and caspase expression in association with apoptosis in failing ischemic sheep left ventricles. Electrophoresis 1999; 20(10): 2046–52
Sawyer DB, Colucci WS. Mitochondrial oxidative stress in heart failure: ‘oxygen wastage’ revisited. Circ Res 2000; 86(2): 119–20
Singh N, Dhalla AK, Seneviratne C, et al. Oxidative stress and heart failure. Mol Cell Biochem 1995; 147(1–2): 77–81
Cadenas E. Biochemistry of oxygen toxicity. Ann Rev Biochem 1989; 58: 79–110
Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 1993; 23(1): 21–48
Shlafer M, Myers CL, Adkins S. Mitochondrial hydrogen peroxide generation and activities of glutathione peroxidase and Superoxide dismutase following global ischemia. J Mol Cell Cardiol 1987; 19(12): 1195–206
Vandeplassche G, Hermans C, Thone F, et al. Mitochondrial hydrogen peroxide generation by NADH-oxidase activity following regional myocardial ischemia in the dog. J Mol Cell Cardiol 1989; 21(4): 383–92
Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation 1997; 96(10): 3264–5
Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000; 86(5): 494–501
Li Z, Bing OH, Long X, et al. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol 1997; 272(5 Pt 2): H2313–9
Chen Q, Tu V, Wu Y, et al. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch Biochem Biophys 2000; 373(1): 242–8
Siwik DA, Tzortzis JD, Pimentai DR, et al. Inhibition of copper-zinc Superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res 1999; 85(2): 147–53
Maulik N, Yoshida T, Das DK. Regulation of cardiomyocyte apoptosis in ischemic reperfused mouse heart by glutathione peroxidase. Mol Cell Biochem 1999; 196(1-2): 13–21
Kotamraju S, Konorev EA, Joseph J, et al. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen: role of reactive oxygen and nitrogen species. J Biol Chem 2000; 275(43): 33585–92
Maulik N, Yoshida T. Oxidative stress developed during open heart surgery induces apoptosis: reduction of apoptotic cell death by ebselen, a glutathione peroxidase mimic. J Cardiovasc Pharmacol 2000; 36(5): 601–8
Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radie Biol Med 2000; 28(1): 141–64
Tribble DL. AHA science advisory. Antioxidant consumption and risk of coronary heart disease: emphasison vitamin C, vitamin E, and beta-carotene: a statement for healthcare professionals from the American Heart Association. Circulation 1999; 99(4): 591–5
Jha P, Flather M, Lonn E, et al. The antioxidant vitamins and cardiovascular disease: a critical review of epidemiologic and clinical trial data. Ann Intern Med 1995; 123(11): 860–72
Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999; 13(9): 1007–24
Boscoboinik D, Szewczyk A, Azzi A. Alpha-tocopherol (vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch Biochem Biophys 1991; 286(1): 264–9
Chojkier M, Houglum K, Solis-Herruzo J, et al. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts: a role for lipid peroxidation? J Biol Chem 1989; 264(28): 16957–62
Pinnel SR, Murad S, Darr D. Induction of collagen synthesis by ascorbic acid: a possible mechanism. Arch Dermatol 1987; 123(12): 1684–6
Sawyer DB, Fukazawa R, Arstall MA, et al. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ Res 1999; 84(3): 257–65
Arstall MA, Sawyer DB, Fukazawa R, et al. Cytokine-mediated apoptosis in cardiac myocytes: the role of inducible nitric oxide synthase induction and peroxynitrite generation. Circ Res 1999; 85(9): 829–40
Ekelund UE, Harrison RW, Shokek O, et al. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res 1999; 85(5): 437–45
Ukai T, Cheng CP, Tachibana H, et al. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing-induced heart failure. Circulation 2001; 103(5): 750–5
Cesselli D, Jakoniuk I, Barlucchi L, et al. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 2001; 89(3): 279–86
Werns SW, Shea MJ, Mitsos SE, et al. Reduction of the size of infarction by allopurinol in the ischemic-reperfused canine heart. Circulation 1986; 73(3): 518–24
Castelli P, Munari M, Riva E. Improvement of cardiac function by allopurinol. J Cardiothorac Vasc Anesth 1997; 11(6): 809–10
Castelli P, Condemi AM, Brambillasca C, et al. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J Cardiovasc Pharmacol 1995; 25(1): 119–25
Sisto T, Paajanen H, Metsa-Ketela T, et al. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann Thorac Surg 1995; 59(6): 1519–23
Rashid MA, William-Olsson G. Influence of allopurinol on cardiac complications in open heart operations. Ann Thorac Surg 1991; 52(1): 127–30
Drexler, H. Nitric oxide synthases in the failing human heart: a doubled-edged sword? Circulation 1999; 99(23): 2972–5
De Mello WC, Danser AH. Angiotensin II and the heart: on the intracrine reninangiotensin system. Hypertension 2000; 35(6): 1183–8
Willenheimer R, Dahlof B, Rydberg E, et al. ATI-receptor blockers in hypertension and heart failure: clinical experience and future directions. Eur Heart J 1999; 20(14): 997–1008
Diez J, Panizo A, Hernandez M, et al. Cardiomyocyte apoptosis and cardiac angiotensin-converting enzyme in spontaneously hypertensive rats. Hypertension 1997; 30(5): 1029–34
Goussev A, Sharov VG, Shimoyama H, et al. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol 1998; 275(2 Pt 2): H626–31
Fortuno MA, Ravassa S, Etayo JC, et al. Overexpression of Bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats: effects of AT1 blockade with losartan. Hypertension 1998; 32(2): 280–6
Asai K, Yang GP, Geng YJ, et al. Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest 1999; 104(5): 551–8
Feuerstein G, Yue TL, Ma X, et al. Novel mechanisms in the treatment of heart failure: inhibition of oxygen radicals and apoptosis by carvedilol. Prog Cardiovasc Dis 1998; 41 (1 Suppl. 1): 17–24
Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000; 101(5): 558–69
Ruffolo RR, Feuerstein GZ. Neurohormonal activation, oxygen free radicals, and apoptosis in the pathogenesis of congestive heart failure. J Cardiovasc Pharmacol 1998; 32 (Suppl. 1): S22–30
Barone FC, Campbell WG, Nelson AH, et al. Carvedilol prevents severe hypertensive cardiomyopathy and remodeling. J Hypertens 1998; 16(6): 871–84
Ruffolo RR, Feuerstein GZ. Carvedilol: preclinical profile and mechanisms of action in preventing the progression of congestive heart failure. Eur Heart J 1998; 19 (Suppl. B):B 19–24
Feuerstein GZ, Bril A, Ruffolo RR. Protective effects of carvedilol in the myocardium. Am J Cardiol 1997; 806(HA): 41L–5L
Yue TL, Ma XL, Wang X, et al. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res 1998; 82(2): 166–74
Yue TL, Ma XL, Gu JL, et al. Carvedilol inhibits activation of stress-activated protein kinase and reduces reperfusion injury in perfused rabbit heart. Eur J Pharmacol 1998; 345(1): 61–5
Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res 2000; 45(3): 560–9
Homcy CJ. Signaling hypertrophy: how many switches, how many wires. Circulation 1998; 97(19): 1890–2
Force T, Pombo CM, Avruch JA, et al. Stress-activated protein kinases in cardiovascular disease. Circ Res 1996; 78(6): 947–53
Minamino T, Yujiri T, Papst PJ, et al. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Natl Acad Sci U S A 1999; 96(26): 15127–32
Andreka P, Zang J, Dougherty C, et al. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res 2001; 88(3): 305–12
Yue TL, Wang C, Gu JL, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res 2000; 86(6): 692–9
Sugden PH, Clerk A. ’stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res 1998; 83(4): 345–52
Ma XL, Kumar S, Gao F, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 1999; 99(13): 1685–91
Malhotra R, Brosius FC. Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem 1999; 274(18): 12567–75
Yaoita H, Ogawa K, Maehara K, et al. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 1998; 97(3): 276–81
Okamura T, Miura T, Takemura G, et al. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res 2000; 45(3): 642–50
Huang JQ, Radinovic S, Rezaiefar P, et al. In vivo myocardial infarct size reduction by a caspase inhibitor administered after the onset of ischemia. Eur J Pharmacol 2000; 402(1-2): 139–42
Fauvel H, Marchetti P, Chopin C, et al. Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am J Physiol Heart Circ Physiol 2001; 280(4): H1608–14
Hoglen NC, Hirakawa BP, Fisher CD, et al. Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J Pharmacol Exp Ther 2001; 297(2): 811–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q.M., Tu, V.C. Apoptosis and Heart Failure. Am J Cardiovasc Drugs 2, 43–57 (2002). https://doi.org/10.2165/00129784-200202010-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129784-200202010-00006