Published online Jan 7, 2008. doi: 10.3748/wjg.14.38
Revised: September 12, 2007
Published online: January 7, 2008
AIM: To study the association of three common ABCB11 and ABCC2 polymorphisms (ABCB11: 1331T>C V444A; ABCC2: 3563T>A V1188E and 4544G>A C1515Y) with intrahepatic cholestasis of pregnancy (ICP) and contraceptive-induced cholestasis (CIC).
METHODS: ABCB11 and ABCC2 genotyping data were available from four CIC patients and from 42 and 33 ICP patients, respectively. Allele-frequencies of the studied polymorphisms were compared with those in healthy pregnant controls and Caucasian individuals. Furthermore, serum bile acid levels were correlated with the presence or absence of the 1331 C allele.
RESULTS: The ABCB11 1331T>C polymorphism was significantly more frequent in cholestatic patients than in pregnant controls: C allele 76.2% (CI, 58.0-94.4) vs 51.3% (CI 35.8-66.7), respectively (P = 0.0007); and CC allele 57.1% (CI 36.0-78.3) vs 20% (CI 7.6-32.4), respectively (P = 0.0065). All four CIC patients were homozygous carriers of the C allele. In contrast, none of the studied ABCC2 polymorphism was overrepresented in ICP or CIC patients. Higher serum bile acid levels were found in carriers of the 1331CC genotype compared to carriers of the TT genotype.
CONCLUSION: Our data support a role for the ABCB11 1331T>C polymorphism as a susceptibility factor for the development of estrogen-induced cholestasis, whereas no such association was found for ABCC2. Serum bile acid and γ-glutamyl transferase levels might help to distinguish ABCB4- and ABCB11-related forms of ICP and CIC.
- Citation: Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, Stieger B, Pauli-Magnus C. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol 2008; 14(1): 38-45
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/38.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.38
Intrahepatic cholestasis of pregnancy (ICP) and oral contraceptive-induced cholestasis (CIC) are two acquired forms of cholestasis, which are observed in otherwise healthy young women with a normal medical history. Both syndromes are rapidly reversible upon discontinuation of the hormonal challenge, which suggests that female sex hormones play a key pathogenic role in these forms of cholestasis[12]. In line with these observations, ICP usually occurs during the third trimester of pregnancy, when serum concentrations of estrogens and progesterone reach their peak[34]. Furthermore, women with ICP and female family members of ICP patients have an increased susceptibility to develop intrahepatic cholestasis under oral contraception[5].
A genetic predisposition for both types of hormonal cholestasis has been suspected based upon the strong regional clustering[6], the higher prevalence in female family members of patients with ICP[57] and the co-incidence with hereditary cases of progressive familial intrahepatic cholestasis[57]. Recently, mutations in the ABCB4 gene that encodes the canalicular phospholipid flippase multidrug resistance protein 3 (MDR3) have been implicated in the development of ICP and CIC in a subset of affected patients[58–12]. MDR3-associated cases of hormonal cholestasis are associated with elevated serum γ-glutamyl transferase (γ-GT) levels in 80% of affected patients, which reflects cholangiocytic damage characteristic of MDR3 dysfunction[11].
In the same study, the majority of ICP women without ABCB4 mutations exhibited normal γ-GT levels[11], which suggests a different pathogenic mechanism in this subset of patients. Dysfunction of the bile salt export pump (BSEP) or the multidrug resistance associated protein 2 (MRP2) have, therefore, been proposed as alternative candidate proteins involved in the pathogenesis of hormonal cholestasis. BSEP constitutes the predominant bile salt efflux system of hepatocytes, which mediates the cellular excretion of numerous conjugated bile salts into the bile canaliculus[13–16]. In contrast, the bilirubin transporter MRP2 is the main driving force for bile-salt-independent bile flow through canalicular excretion of reduced glutathione[1718]. Given their important roles in bile formation and bilirubin secretion, inherited and acquired dysfunction of these proteins can lead to severe cholestatic syndromes and conjugated hyperbilirubinemia, respectively[19–21].
In hormonal cholestasis, in vitro inhibition of BSEP by estrogen and progesterone metabolites has been proposed as an underlying pathophysiological mechanism[22]. BSEP inhibition by estrogen and progesterone metabolites takes place from the luminal side of the bile canaliculus (so-called trans inhibition), which requires previous MRP2-mediated canalicular secretion of conjugated metabolites[2223]. Therefore, MRP2 dysfunction might contribute to this form of cholestasis. While sequencing of ABCB11 in unrelated ICP women has not revealed the presence of disease-causing BSEP mutations[11], only little attention has so far been paid to the possible pathogenic role of functional ABCB11 and ABCC2 polymorphisms. Recent observations have suggested that a non-synonymous polymorphism in exon 13 of the ABCB11 gene (1331T>C) is overrepresented in drug-induced cholestatic liver injury[24]. The same polymorphism has recently been observed more frequently in ICP women compared to healthy controls, pointing towards a possible role of this polymorphism as a susceptibility factor for ICP and CIC[25]. Furthermore, two non-synonymous ABCC2 polymorphisms (V1188E and C1515Y) showed significant differences in hepatic MRP2 expression levels compared to the wildtype sequence, which could be relevant for the extent of BSEP trans inhibition[25].
The aim of the present study was, therefore, threefold: (1) to compare allele frequencies of the aforementioned ABCB11 and ABCC2 polymorphisms in a prospectively recruited group of patients with ICP and CIC; (2) to define the relative risk of the different polymorphisms for the development of ICP; and (3) to determine the extent of the increase in serum bile acid levels as marker of cholestasis in the presence of the different ABCB11 1331T>C genotypes.
After approval by the Ethics Committee of the University Hospital of Zurich and written informed consent from all participating individuals, blood samples for DNA extraction were obtained from Caucasian patients with ICP or CIC. The total population of analyzed individuals consisted of two different groups: 25 patients (21 ICPnew patients and four CIC patients) were prospectively recruited for this study, and a second group of 20 patients (ICPold) had already been described in a previous study by Pauli-Magnus and coworkers[11].
Two hundred and five Caucasian volunteers and patients without cholestasis, as well as Caucasian women with uneventful pregnancies (n = 40), served as a control population for BSEP (ABCB11) and MDR3 (ABCB4) genetic variants. These controls have already been described in previous studies[112526]. Specifically, pregnant controls were all healthy, as defined by normal serum levels of transaminases, bilirubin, γ-GT, alkaline phosphatase (AP) and bile acids. Caucasian controls from the study of Pauli-Magnus and coworkers[26] (n = 95) were healthy volunteers recruited for participation in phaseIstudies, with uneventful medical history and normal blood biochemistry. Neither of these two control groups took any regular medication. In the case of the Caucasian control population of Meier and coworkers[25] (n = 110), most patients suffered from extrahepatic malignancies, and cholestatic disease was excluded in all patients. Furthermore, none of these patients used medication known to be associated with the development of cholestasis.
For lack of DNA availability, only 110 out of 205 Caucasian controls could be used for MRP2 sequencing. For the same reason, a new group of Caucasian women with uneventful pregnancies (n = 42) had to be collected for the MRP2 variants. Demographic data and pregnancy course of these women did not differ from the previous control group.
Diagnosis of ICP was based upon: (1) a clinical history of pruritus, which occurred in the third trimester of pregnancy; (2) the presence of laboratory abnormalities suggestive of ICP: fasting serum bile acid ≥ 1.5 ULN (upper limit of normal) and/or serum AP levels ≥ 1.5 ULN and/ or alanine aminotransferase (ALT) levels ≥ 1.5 ULN; and (3) spontaneous resolution of clinical symptoms and laboratory findings after delivery. Diagnosis of CIC was based upon laboratory abnormalities as defined for ICP and the exclusion of preexisting liver disease defined by: (1) a negative serology for hepatitis A, B and C; (2) the exclusion of other preexisting medical conditions that could explain liver injury, such as congestive heart failure, systemic infection, or malignancy; (3) normal liver ultrasound; and (4) a clear causal relationship to drug intake. Each case of ICP and CIC was evaluated by at least one obstetrician and one hepatologist, as well as by a clinical pharmacologist.
Full length ABCB4 and ABCB11 sequencing data were already available from the control groups, as well as from ICPold patients. To allow detection of additional ABCB4 and ABCB11 mutations in the new group, complete sequencing of these two genes was also performed in the 25 newly recruited patients. Genotyping of ABCC2 included all CIC patients, as well as 17 out of 21 patients from the ICPnew group and 16 of 21 patients in the ICPold group, which yielded a total number of 33 patients for ABCC2 genotyping. In nine patients (four ICPnew and five ICPold), no ABCC2 genotyping could be performed for lack of DNA availability.
Isolation of DNA and DNA sequencing was done at Epidauros Biotechnology AG, Bernried, Germany. Genomic and cDNA sequences were derived from known sequences (ABCB4: AC005068.2 for non-coding exons -3 to 1 and coding exons 2 and 3; AC006154.1 for exons 4 to 12; AC0005045.2 for exons 13 to 28; and NM_000443.2 for cDNA; ABCB11: GenBank accession number AC008177.3 for promoter and exons 1 to 21; AC069165.2 for exons 22 to 28 and NM_003742.2 for cDNA).
ABCB4 and ABCB11: Sequencing of ABCB4 covered a proximately 8000 bp, including (1) 2000 bp of the upstream promoter region and non-coding exon -3 to 1 and, (2) coding exons 2-28 and 100-350 bp of the intronic sequence around each exon. For ABCB11, sequencing covered 10 000 bp including (1) non-coding exon 1 and 2400 bp of the upstream promoter region and, (2) coding exons 2-28 and 100-350 bp of the intronic sequence around each exon. Primers for genomic DNA were designed to span all exons and at least 100 bp of the flanking intronic sequence at the 5’ and 3’ end of each exon. The DNA sequence of purified PCR fragments was analyzed on an ABI3700 capillary sequencer (ABI, Weiterstadt, Germany) and assembled using the phredPhrap, Consed and PolyPhred software (University of Washington). Details regarding the primers, optimized PCR conditions and subsequent purification and sequencing of the fragments are available at info@epidauros.com.
ABCC2: Three non-synonymous polymorphisms with a potential impact on MRP2 function and expression were chosen for genotyping[25]: 1249G>A variant (V417I, rs2273697), 3563T>A (V1188E, rs17222723) and 4544G>A (C1515Y, rs8187710). Genotyping was performed with the Custom TaqMan SNP Genotyping Assays procedure (Applied Biosystems, Foster City, CA, USA) which contained a sense- and an antisense primer and two probes, labeled with fluorescent reporter dyes, either VIC or 6-Fam at the 5’ end and a non-fluorescent quencher at the 3’ end to distinguish between alleles 1 and 2, respectively. Primer and probe sequences for individual SNPs are given in Table 1. Probe solution (0.625 &mgr;L) and 12.5 &mgr;L of 2 × Universal PCR Master Mix (Applied Biosystems) were brought to 25 &mgr;L with 20 ng of genomic DNA. PCR reaction (2 min at 50°C, followed by 10 min at 95°C and 40 cycles of 15 s at 92°C and 1 min at 60°C). Allelic discrimination was processed with an ABI PRISM 7700 Sequence Detector.
| cDNA position1 | SNP | Exon | Amino acid change | Eense-/antisense primer | Probes2 |
| 1249 | G>A | 10 | V417I | 5’-CCAACTTGGCCAGGAAGGA-3’/ | VIC 5’-CTGTTTCTCCAACGGTGTA-3’ |
| 5’-GGCATCCACAGACATCAGGTT-3’ | FAM 5’-ACTGTTTCTCCAATGGTGTA-3’ | ||||
| 3563 | T>A | 25 | V1188E | 5’-GCACCAGCAGCGATTTCTG-3’/ | VIC 5’-ACACAATGAGGTGAGGAT-3’ |
| 5’-AGGTGATCCAGGAAAAGACACATTT-3’ | FAM 5’-ACAATGAGGAGAGGAT-3’ | ||||
| 4544 | G>A | 32 | C1515Y | 5’-GTAATGGTCCTAGACAACGGGAAG-3’/ | VIC 5’-AGAGTGCGGCAGCC-3’ |
| 5’-CCAGGGATTTGTAGCAGTTCTTCAG-3’ | FAM 5’-ATTATAGAGTACGGCAGCC-3’ |
Genotype distribution, allelic frequencies and odds ratios (ORs) are given with 95% CI. In ABCB11, formal statistical analysis was only performed for the 1331T>C polymorphisms (rs2287622), whereas for ABCC2 analysis, it included two highly linked polymorphisms. No correction according to Bonferroni was, therefore, required. Differences investigated in our study apply to a proportion of diseased versus non-diseased individuals within the whole population, using an unmatched case control design. Response (ICP versus non-ICP) and predictors (T versus C) were both binary variables and were therefore best condensed into a 2 × 2 table. Differences in genotype distribution between patients and controls were calculated with the χ2 test, and difference in allelic frequencies between two groups was performed using a 2 × 2 Fisher exact test. P ≤ 0.05 was considered statistically significant.
A total of 25 unrelated patients with estrogen-associated intrahepatic cholestasis were prospectively enrolled in this study, 21 with ICP and four with oral CIC. Demographic data and laboratory findings in ICP patients are given in Table 2. Only two patients showed elevated γ-GT levels > 1.5 ULN, while total bile acid levels were elevated in all patients in whom it was determined (16 out of 21; range, 1.7-17.3 ULN). Three patients had a previous history of ICP; three pregnancies were twin pregnancies, and one patient experienced cholestasis under previous oral contraception.
| Patient ID | Age (yr) | Liver parameters | Comments | Genotypes of SNPs | |||||||
| ALT (ULN) | AP (ULN) | γ-GT (ULN) | tBili (ULN) | tBA (ULN) | No of preg/No ICP | Others | ABCB111331T>C (V444A) | ABCC23600T>A (V1188E) | ABCC24581G>A (C1515Y) | ||
| 1 | 36 | 0.9 | 2.3 | 3.3 | 0.8 | 10.7 | 2/1 | CC | TA | GA | |
| 2 | 31 | 1.6 | 2.5. | 0.3 | 0.8 | 17.3 | 3/2 | TC | TA | GA | |
| 3 | 35 | 8.9 | 2.3 | 1 | 0.5 | 10 | 1/1 | TC | TT | GG | |
| 4 | 29 | 1.2 | 3 | 0.6 | 0.5 | 7 | 2/1 | CC | TT | GG | |
| 5 | 42 | 11.6 | 1.4 | 0.4 | 0.8 | 6 | 1/1 | TC | TT | GG | |
| 6 | 28 | 4.8 | 1.8 | 0.2 | 0.8 | 4.7 | 2/1 | Twins | CC | TA | GA |
| 7 | 32 | 11.9 | 3 | 1.2 | 0.9 | 3.6 | nd | CC | TT | GG | |
| 8 | 16 | 5.2 | 2 | 0.4 | nd | 3.3 | nd | TC | TT | GG | |
| 9 | 38 | 6.2 | 1.2 | 1.1 | 0.4 | 3 | 2/1 | TC | TT | GG | |
| 10 | 23 | 1.6 | 1.3 | 1.3 | 0.8 | 2.4 | 1/1 | Twins | TC | TT | GG |
| 11 | 30 | 0.4 | 0.9 | 0.2 | 0.5 | 2.4 | 3/1 | TC | TT | GG | |
| 12 | 22 | 0.3 | 1.8 | 0.1 | 0.5 | 2.1 | nd | TC | TA | GA | |
| 13 | 32 | 0.2 | 0.9 | 0.7 | 0.2 | 2 | 2/1 | TT | TT | GG | |
| 14 | 30 | 0.8 | 1.2 | 0.3 | 0.6 | 1.7 | 1/1 | CC | TT | GG | |
| 15 | 28 | 7.5 | 2.3 | 0.9 | 0.6 | nd | nd | TC | TT | GG | |
| 16 | 31 | 0.9 | 2.1 | 1.2 | 0.5 | 8.1 | 1/1 | CC | TT | GG | |
| 17 | 20 | 3.4 | 1.1 | 0.4 | 0.5 | 2.6 | 1/1 | TT | T | GG | |
| 18 | 24 | 7.2 | 1.3 | 0.4 | 0.8 | nd | 1/1 | CC | nd | nd | |
| 19 | 31 | 8.9 | 1.3 | 1.2 | 1.5 | nd | 2/2 | CC | nd | nd | |
| 20 | 32 | 11 | 2.2 | 0.4 | 3.1 | nd | 3/3 | Pruritus with contraceptives | CC | n | nd |
| 21 | 41 | 10.9 | 2.9 | 5.7 | 1.2 | nd | 1/1 | Twins | CC | nd | nd |
| Summary median (Q1; Q3) | 31 (28; 32) | 4.8 (0.9; 8.9) | 1.8 (1.2; 2.3) | 0.6 (0.4; 1.2) | 0.6 (0.5; 0.8) | 3.5 (2.4; 7.3) | |||||
Characteristics of patients with CIC are given in Table 3. One patient showed elevated γ-GT levels. Total bile acid levels were elevated in all three patients in whom it was determined (three out of four; range, 1.6-22.3 ULN). Oral contraceptive preparations used in the four patients contained comparable amounts of ethinylestradiol (20-35 &mgr;g) while the progesterone-like portion ranged from 50 to 150 &mgr;g. All patients had a liver biopsy done for strictly diagnostic reasons, which showed intrahepatic cholestasis in three patients. One patient had a previous history of ICP.
| Patient ID | Oral contraceptive | Age (yr) | Exposure time | Liver parameters | Comments | Genotypes of SNPs | |||||||
| ALT | AP | γGT | tBili | tBA | Clinical features | Histology | ABCB111331T>C (V444A) | ABCC23600T>A (V1188E) | ABCC24581G>A (C1515Y) | ||||
| (ULN) | (ULN) | (ULN) | (ULN) | (ULN) | |||||||||
| 11 | 30 &mgr;g ethinylestradiol/75 &mgr;g gestodene | 32 | nd | 4.9 | 1.7 | 1 | 10.9 | 22.3 | Jaundice | Intrahepatic cholestasis | CC | TT | GG |
| 2 | 30 &mgr;g ethinylestradiol/150 &mgr;g levonorgestrel | 15 | 21 d | 1 | 3 | 1 | 4.2 | nd | Jaundice, nausea, pruritus | Extensive intrahepatic cholestasis | CC | TT | GG |
| 3 | 35 &mgr;g ethinylestradiol/50 &mgr;g levonorgestrel | 40 | 2 yr | 3.9 | 2.8 | 3.6 | 0.5 | 1.6 | Pruritus | Bland | CC | TT | GG |
| 4 | 35 &mgr;g ethinylestradio/2 mg cyproteron | 34 | nd | 1 | 1.3 | nd | 2.8 | 1.6 | Jaundice | Extensive canalicular cholestasis, mild portal inflamation | CC | TA | GA |
ABCB4 and ABCB11: Sequence analysis in the 25 newly recruited patients with estrogen-associated cholestasis revealed no disease-associated non-synonymous mutations in ABCB4 or ABCB11. Furthermore, in line with previous findings[11], no ABCB4 polymorphism was found to be overrepresented in the ICP and CIC groups compared to pregnant women without cholestasis and healthy Caucasian individuals. All of the detected genetic variants in ABCB11 and ABCB4 were in Hardy Weinberg equilibrium.
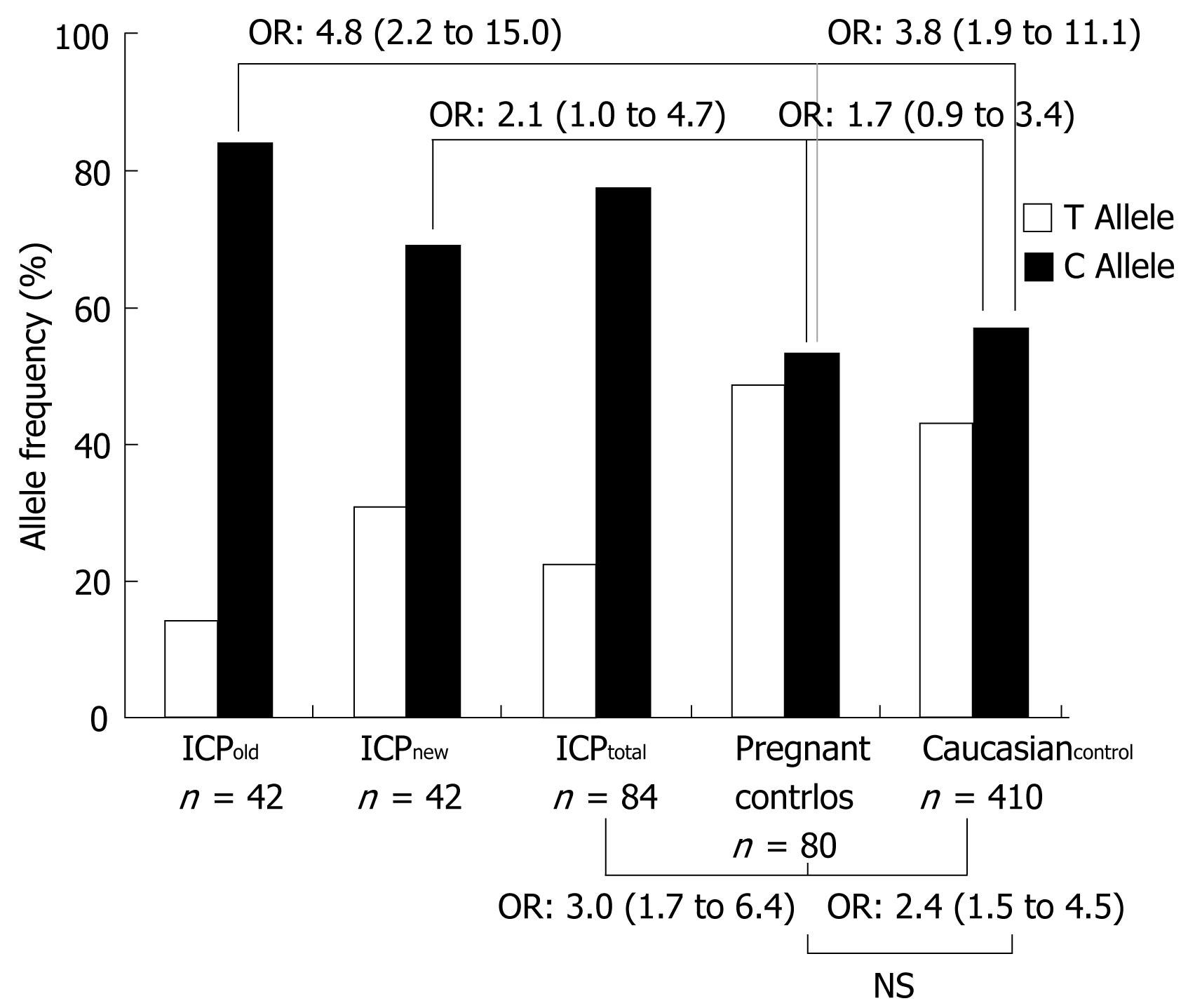
In contrast, the ABCB11 1331T>C → V444A polymorphism was significantly more frequent in ICP and CIC patients compared to the two control groups. Specifically, the CC genotype was encountered in 57.1% of all ICP patients (ICPnew, 47.6% and ICPold, 67.7%) and 100% of CIC patients compared to 20 and 32.2% in pregnant women without cholestasis and healthy Caucasian controls, respectively (Table 4). In line with these findings, the ORs of C versus T were 3.0 (1.7-6.4) for all ICP patients (ICPnew + ICPold) versus healthy pregnant control women (ICPnew, 2.1; 1.0-4.7 and ICPold 4.8; 2.2-15.0) (Table 5 and Figure 1). With the exception of this polymorphism and two intronic variants that were found to be closely linked to the 1331T>C polymorphism in previous studies [intron 13: (+70) C>T and intron 14 (+32) T>C][26], the allele frequency of the remaining common variants in the patients with ICP and CIC was comparable to that observed in healthy pregnant and Caucasian controls. Due to the small sample size, no significance levels could be calculated for the CIC group. However, all patients in this group were homozygous for the C at position 1331, which is highly suggestive of an overrepresentation of this allele compared to the control groups.
| Genotype SNP | ICPold | ICPnew | ICPtotal | Pregnant controls | Caucasian controls | |||||
| n(%) | 95 % CI | n(%) | 95 % CI | n(%) | 95 % CI | n(%) | 95 % CI | n(%) | 95 % CI | |
| ABCB11 1331T>C (V444A) | 21 (100) | 21 (100) | 42 (100) | 40 (100) | 205 (100) | |||||
| TT (VV) | - | - | 2 (9.5) | 0.0-22.1 | 2 (4.8) | 0.0-13.9 | 7 (17.5) | 5.7-29.3 | 38(18.5) | 13.2-23.9 |
| CC (AA) | 14 (67.7) | 46.5 -86.8 | 10 (47.6) | 26.3-69.0 | 24 (57.1) | 36.0-78.3 | 8 (20) | 7.6-32.4 | 66 (32.2) | 25.8-38.6 |
| TC (VA) | 7 (33.3) | 13.2-53.5 | 9 (42.9) | 21.7-64.0 | 16 (38.1) | 17.3-58.9 | 25 (62.5) | 47.5-77.5 | 101 (49.3) | 42.4-56.1 |
| Frequency C allele | 35 (83.3) | 67.4-99.3 | 29 (69.0) | 49.3-88.8 | 64 (76.2) | 58.0-94.4 | 41 (51.3) | 35.8-66.7 | 233 (56.8) | 50.1-63.6 |
| Frequency T allele | 7 (16.7) | 0.7-32.6 | 13 (31.0) | 11.2-50.7 | 20 (23.8) | 5.6-42.0 | 39 (48.8) | 33.3-64.2 | 177 (43.2) | 36.4-50.0 |
| ABCC2 3563T>A (V1188E) | 16 (100) | 17 (100) | 33 (100) | 42 (100) | 110 (100) | |||||
| TT (VV) | 15 (93.8) | 71.3-98.6 | 13 (76.5) | 52.3-90.4 | 28 (84.8) | 68.9-93.3 | 37 (88.1) | 74.3-96.1 | 95 (86.4) | 68.9-93.3 |
| AA (EE) | - | - | - | - | - | - | - | - | 1 (0.9) | 0.0-5.0 |
| TA (VE) | 1 (3.1) | 0.0-15.8 | 4 (23.5) | 9.6-47.7 | 5 (15.2) | 6.7-31.1 | 5 (11.9) | 3.9-25.7 | 14 (12.7) | 7.1-20.5 |
| ABCC2 4544G>A (C1515Y) | 16 (100) | 17 (100) | 33 (100) | 42 (100) | 110 (100) | |||||
| GG (CC) | 15 (93.8) | 71.3-98.6 | 13 (76.5) | 52.3-90.4 | 28 (84.8) | 68.9-93.3 | 36 (85.7) | 71.4-94.6 | 95 (86.4) | 68.9-93.3 |
| AA (YY) | - | - | - | - | - | - | - | - | 1 (0.9) | 0.0-5.0 |
| GA (CY) | 1 (3.1) | 0.0-15.8 | 4 (23.5) | 9.6-47.7 | 5 (15.2) | 6.7-31.1 | 6 (14.3) | 5.4-28.6 | 14 (12.7) | 7.1-20.5 |
| CC vs TT | C vs T | |||||
| Fisher | Odds ratio | 95% CI | Fisher | Odds ratio | 95% CI | |
| ICPoldvs Pregnant controls | 0.0041 | nd1 | - | 0.0004 | 4.8 | 2.2-15.0 |
| ICPoldvs Caucasian controls | 0.0029 | nd1 | - | 0.0005 | 3.8 | 1.9-11.1 |
| ICPnewvs Pregnant controls | 0.1082 | 4.4 | 0.7-27.2 | 0.0441 | 2.1 | 1.0-4.7 |
| ICnewvs Caucasian controls | 0.1461 | 2.9 | 0.6-13.8 | 0.0850 | 1.7 | 0.9-3.4 |
| ICPtotalvs Pregnant controls | 0.0065 | 10.5 | 1.9-63.7 | 0.0007 | 3.0 | 1.7-6.4 |
| ICPtotalvs Caucasian controls | 0.0025 | 6.9 | 1.6-32.1 | 0.0006 | 2.4 | 1.5-4.5 |
ABCC2: The 1249G>A polymorphism was not found in our patients. In contrast, 3563T>A and 4544G>A were strongly linked and distributed similarly in all groups (Table 4). No significant difference in the frequency of these two polymorphisms was observed between affected ICP and CIC patients and healthy controls. Heterozygous carriers of the 3563A and the 4544A alleles were found in 15.2% of ICP patients (ICPnew, 23.5% and ICPold, 3.1%) compared to 11.9 and 12.7% in pregnant women without cholestasis and healthy Caucasian controls, respectively (Table 4). Furthermore, one CIC patient was a heterozygous carrier for the two variant alleles at positions 3563 and 4544.
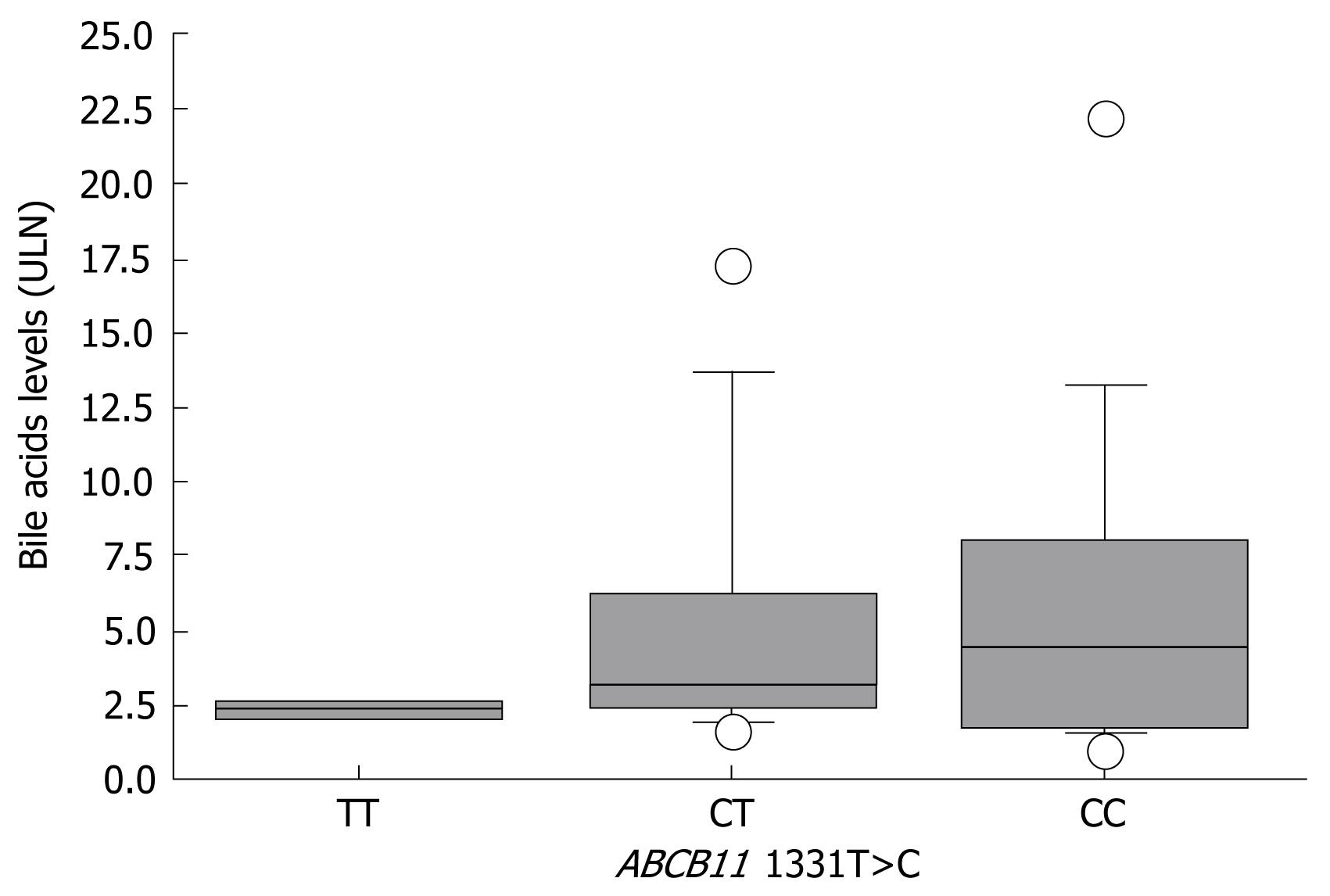
For correlation of bile acid levels with the corresponding genotype at position 1331 of ABCB11, ICP and CIC samples were analyzed together. Bile acid levels were available for 16 out of 21 ICPnew patients, seven out of 20 ICPold patients, and three out of four CIC patients, which yielded a total of 26 samples (CC, 14 patients; CT, 10 patients; and TT, two patients). Interindividual variability in serum bile acid levels was high and ranged from 1.7 to 22.3 ULN and 1.7 to 17.3 ULN in CC and CT patients, respectively. Serum bile acid levels gradually increased from carriers of the TT genotype to carriers of the CC genotype, with medians of 2.3 ULN (Q1, 2.2; Q3, 2.5), 3.2 ULN (Q1, 2.4; Q3, 6.2) and 4.4 ULN (Q1, 2.2; Q3, 7.8) for TT, TC and CC, respectively (Figure 2).
We investigated the risk association between different ABCB11 and ABCC2 polymorphisms in ICP and CIC, and correlated different genotypes with serum bile acid levels as a marker of cholestasis. In our group of 25 patients with estrogen-associated cholestasis (21 with ICP and four with CIC), there was a highly significant association between the presence of the C allele at position 1331 of ABCB11 and the presence of cholestasis, which confirms preliminary results from another collective of ICP women, in whom such an overrepresentation was first observed[11]. While in vitro function of both BSEP variants, as measured by taurocholate transport activity, is comparable[24], BSEP expression in healthy liver tissue of Caucasian individuals has recently been found to be lower in carriers of the 1331C allele[25]. Such differences in hepatic BSEP expression levels might offer one valuable explanation for the increased susceptibility to the development of cholestasis under specific circumstances, such as hormonal challenges. Furthermore, serum bile acids as a marker of in vivo BSEP function was influenced by the underlying genotype. Lowest bile acid levels were observed in patients with the TT genotype and highest levels in carriers of the CC genotype. Although the accepted level of statistical significance was not reached due to high interindividual variability, this observation is in line with the hypothesis that the underlying genotype at position 1331 is a determinant of BSEP function, and hence contributes to the individual risk of developing cholestasis.
The homozygous state for the 1331T>C polymorphism has only recently been observed in a very severe case of pregnancy-associated cholestasis with serum bile acid levels > 40-fold above the ULN. Interestingly, decreased BSEP expression levels were found in a liver biopsy obtained from this patient[27]. Although this patient carried an additional ABCB4 mutation, the presence of decreased hepatic BSEP expression and highly elevated bile acid levels strongly support a BSEP-related mechanism as a predominant pathogenetic factor. The same patient also developed severe cholestasis under previous use of oral contraceptives, which supports the notion that the same polymorphism also predisposes to oral CIC.
In our group, seven patients (from ICPold) carried additional ABCB4 mutations, while no such mutations were detected in the remaining 35 out of 42 ICP patients (ICPold, 14 and ICPnew, 21). This finding suggests that the ABCB11 1331T>C polymorphism independently contributes to an individual’s risk for developing cholestasis under certain conditions. On the other hand, it can be speculated whether the combination of the 1331T>C polymorphism with ABCB4 mutations might be a risk constellation for a severe disease course, as observed by Keitel and coworkers[27].
In contrast, no association was found between the presence of the non-synonymous polymorphisms at positions 1188 and 1515 of MRP2 and the presence of ICP or CIC. A possible pathogenic role of these two polymorphisms in ICP and CIC was suspected based upon the genotype-dependent alteration in hepatic MRP2 expression levels in healthy human liver tissue[25]. Specifically, heterozygous carriers of the glutaminic acid at position 1188 and tyrosine at position 1551 showed significantly higher levels of MRP2 in their liver than homozygous carriers of valine and cysteine, respectively[25]. As BSEP inhibition by estrogen and progesterone metabolites requires prior MRP2-mediated secretion into the bile canaliculus, high MRP2 expression was suspected as a risk factor for the development of estrogen-dependent cholestasis[22].
Several conclusions can be drawn from this study. First, our data point toward a pathogenic relevance of the ABCB11 1331T>C polymorphism in ICP and CIC. While these types of cholestasis are so far mainly attributed to different disease-causing mutations in ABCB4[51112], our data support a clear association between the presence of a frequent ABCB11 polymorphism and ICP. Interestingly, all of the patients with CIC were homozygous carriers of the C allele at position 1331. It can be speculated that lower estrogen levels in CIC compared to second or third trimester pregnancy require two low-function alleles to result in cholestasis. Furthermore, the 1331T>C variant was also found to be associated with other inherited and acquired forms of cholestasis, such as benign recurrent intrahepatic cholestasis and drug-induced cholestasis[242829]. This suggests a role for this polymorphism as a risk factor for different cholestatic conditions, which have so far been regarded as different disease entities[2030].
Second, while γ-GT levels are elevated in ICP patients who carried a disease-causing ABCB4 mutation[11], serum bile acid levels are influenced by the BSEP genotype at position 444 of ABCB11. It can, therefore, be speculated that these two parameters allow us to clinically distinguish between MDR3- and BSEP-related forms of estrogen-related cholestasis, as it is already done for progressive forms of inherited familial intrahepatic cholestasis[521]. From a prognostic point of view, this might help to distinguish patients that carry a common susceptibility factor from those who carry a disease-causing ABCB4 mutation, which in some cases, has been associated with disease progression[7111231]. Third, although a pathogenic involvement of MRP2 in estrogen-induced cholestasis has longly been suspected, common ABCC2 polymorphisms have not been associated with the development of cholestasis. We did not exclude the presence of disease-associated ABCC2 mutations in our group, but normal bilirubin levels in all but one patient suggests no major MRP2 dysfunction, which should result in a Dubin Johnson phenotype[32].
In summary, our data support a role for the ABCB11 1331T>C polymorphism as a susceptibility factor for the development of estrogen-induced cholestasis, whereas no such association was found for ABCC2. Serum bile acid and γ-GT levels might help to distinguish ABCB4 and ABCB11-related forms of ICP and CIC.
Intrahepatic cholestasis of pregnancy (ICP) and oral contraceptive-induced cholestasis (CIC) are two acquired forms of cholestasis, which are observed in otherwise healthy young women with a normal medical history. The bile salt export pump (BSEP, ABCB11) and the multidrug resistance protein 2 (MRP2, ABCC2) might be of pathogenetic importance in both conditions.
A genetic predisposition for both types of hormonal cholestasis has been suspected based upon the strong regional clustering, the higher prevalence in female family members of patients with ICP, and the co-incidence with hereditary cases of progressive familial intrahepatic cholestasis. While mutations in the ABCB4 gene that encodes the canalicular phospholipid flippase multidrug resistance protein 3 (MDR3) have been implicated in the development of ICP and CIC in a subset of affected patients, the role of genetic variants in ABCB11 and ABCC2 remains unclear.
Our data support a role of the ABCB11 1331T>C polymorphism as a susceptibility factor for the development of estrogen-induced cholestasis, whereas no such association was found for ABCC2. Serum bile acid and γ-GT levels might help to distinguish ABCB4- and ABCB11-related forms of ICP and CIC.
While the clinical consequences of such findings are still uncertain at this time, they provide important new insights in the role of genetically determined differences in canalicular transporter expression and function for the development of estrogen-induced cholestasis. In the future, the integration of different factors that predict cholestasis might be used to counsel pregnant patients or to avoid certain medications in susceptible patients.
ICP: Intrahepatic cholestasis of pregnancy; CIC: contraceptive-induced cholestasis; BSEP: Bile Salt Export Pump (ABCB11); MRP2: Multidrug Resistance Protein 2 (ABCC2); MDR3: Multidrug Resistance Protein 3 (ABCB4).
The study characterized a potential underlying defect in the subgroup of normal γ-GT ICP patients and contributes to a clinical risk assessment for the future. This study from a group with longstanding experience in transporter genomics is well designed and presented in a clearly written manuscript.
| 1. | Bacq Y, Sapey T, Brechot MC, Pierre F, Fignon A, Dubois F. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364. [Cited in This Article: ] |
| 2. | Kenyon AP, Piercy CN, Girling J, Williamson C, Tribe RM, Shennan AH. Obstetric cholestasis, outcome with active management: a series of 70 cases. BJOG. 2002;109:282-288. [Cited in This Article: ] |
| 3. | Gonzalez MC, Reyes H, Arrese M, Figueroa D, Lorca B, Andresen M, Segovia N, Molina C, Arce S. Intrahepatic cholestasis of pregnancy in twin pregnancies. J Hepatol. 1989;9:84-90. [Cited in This Article: ] |
| 4. | Reyes H, Sjovall J. Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Ann Med. 2000;32:94-106. [Cited in This Article: ] |
| 5. | Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, Scheffer GL, Paul M, Burdelski M, Bosma PJ. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448-1458. [Cited in This Article: ] |
| 6. | Reyes H, Gonzalez MC, Ribalta J, Aburto H, Matus C, Schramm G, Katz R, Medina E. Prevalence of intrahepatic cholestasis of pregnancy in Chile. Ann Intern Med. 1978;88:487-493. [Cited in This Article: ] |
| 7. | Leevy CB, Koneru B, Klein KM. Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease. Gastroenterology. 1997;113:966-972. [Cited in This Article: ] |
| 8. | Gendrot C, Bacq Y, Brechot MC, Lansac J, Andres C. A second heterozygous MDR3 nonsense mutation associated with intrahepatic cholestasis of pregnancy. J Med Genet. 2003;40:e32. [Cited in This Article: ] |
| 9. | Lucena JF, Herrero JI, Quiroga J, Sangro B, Garcia-Foncillas J, Zabalegui N, Sola J, Herraiz M, Medina JF, Prieto J. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037-1042. [Cited in This Article: ] |
| 10. | Mullenbach R, Linton KJ, Wiltshire S, Weerasekera N, Chambers J, Elias E, Higgins CF, Johnston DG, McCarthy MI, Williamson C. ABCB4 gene sequence variation in women with intrahepatic cholestasis of pregnancy. J Med Genet. 2003;40:e70. [Cited in This Article: ] |
| 11. | Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, Zimmermann R, Kenngott S, Beuers U, Reichel C. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91-102. [Cited in This Article: ] |
| 12. | Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353:210-211. [Cited in This Article: ] |
| 13. | Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649-1658. [Cited in This Article: ] |
| 14. | Noe J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123:1659-1666. [Cited in This Article: ] |
| 15. | Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322-342. [Cited in This Article: ] |
| 16. | Meier PJ, Stieger B. Molecular Mechanisms in Bile Formation. News Physiol Sci. 2000;15:89-93. [Cited in This Article: ] |
| 17. | Keppler D, Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis. 2000;20:265-272. [Cited in This Article: ] |
| 18. | Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch. 2007;453:643-659. [Cited in This Article: ] |
| 19. | Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370-1379. [Cited in This Article: ] |
| 20. | Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, Mullhaupt B, Meier PJ, Pauli-Magnus C. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43:536-543. [Cited in This Article: ] |
| 21. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. [Cited in This Article: ] |
| 22. | Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422-430. [Cited in This Article: ] |
| 23. | Vallejo M, Briz O, Serrano MA, Monte MJ, Marin JJ. Potential role of trans-inhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157. [Cited in This Article: ] |
| 24. | Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47-60. [Cited in This Article: ] |
| 25. | Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62-74. [Cited in This Article: ] |
| 26. | Pauli-Magnus C, Kerb R, Fattinger K, Lang T, Anwald B, Kullak-Ublick GA, Beuers U, Meier PJ. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779-791. [Cited in This Article: ] |
| 27. | Keitel V, Vogt C, Haussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology. 2006;131:624-629. [Cited in This Article: ] |
| 28. | Kubitz R, Keitel V, Scheuring S, Kohrer K, Haussinger D. Benign recurrent intrahepatic cholestasis associated with mutations of the bile salt export pump. J Clin Gastroenterol. 2006;40:171-175. [Cited in This Article: ] |
| 29. | Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47-60. [Cited in This Article: ] |
| 30. | Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43:342-357. [Cited in This Article: ] |
| 31. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282-287. [Cited in This Article: ] |
| 32. | Borst P, Zelcer N, van de Wetering K. MRP2 and 3 in health and disease. Cancer Lett. 2006;234:51-61. [Cited in This Article: ] |