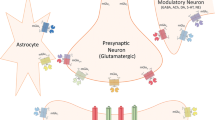
Abstract
Background
Positive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) modulators enhance glutamate transmission via the AMPA receptor by altering the rate of desensitization; alone they have no intrinsic activity. They are the only class of compounds known that may pharmacologically separate AMPA subtypes.
Objective
This manuscript will review preclinical work on positive AMPA modulators, with clinical examples where relevant.
Results
The activity of these compounds appears to be determined by the AMPA receptor subunit composition. Studies have shown that splice variant and/or subunit combinations change the desensitization rate of this receptor. Also, these subunits are heterogeneously expressed across the central nervous system. Therefore, the functional outcome of different positive AMPA modulators could indeed be different. The origins of this pharmacological class come from hippocampal long-term potentiation studies, so quite naturally they were first studied in models of short- and long-term memory (e.g., delayed match to sample, maze performance). In general, these agents were procognitive. However, more recent work with different chemical classes has suggested additional therapeutic effects in models of schizophrenia (e.g., amphetamine locomotor activity), depression (e.g., forced swim test), neuroprotection (e.g., NMDA agonist lesions) and Parkinson’s disease (e.g., 6-hydroxydopamine lesion).
Conclusions
In conclusion, positive modulation of AMPA may offer numerous therapeutic avenues for central nervous system drug discovery.
Similar content being viewed by others
References
Anis NA, Berry SC, Burton NR, Lodge D (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79(2):565–575
Arai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G (1994) A centrally active drug that modulates AMPA receptor gated currents. Brain Res 638(1–2):343–346
Arai A, Guidotti A, Costa E, Lynch G (1996) Effect of the AMPA receptor modulator IDRA 21 on LTP in hippocampal slices. NeuroReport 7(13):2211–2215
Arai AC, Xia YF, Suzuki E (2004) Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience 123(4):1011–1024
Bai F, Li X, Clay M, Lindstrom T, Skolnick P (2001) Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav 70(2–3):187–192
Bartolini L, Risaliti R, Pepeu G (1992) Effect of scopolamine and nootropic drugs on rewarded alternation in a T-maze. Pharmacol Biochem Behav 43(4):1161–1164
Baumbarger PJ, Muhlhauser M, Zhai J, Yang CR, Nisenbaum ES (2001) Positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in prefrontal cortical pyramidal neurons by a novel allosteric potentiator. J Pharmacol Exp Ther 298(1):86–102
Beneyto M, Meador-Woodruff JH (2004) Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol 468(4):530–554
Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Vicini S, Costa E (1993) Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus. Recept Channels 1(4):267–278
Bigge CF, Nikam SS (1997) AMPA receptor agonists, antagonists and modulators—their potential for clinical utility. Expert Opin Ther Pat 7(10):1099–1114
Black MD, Wotanis J, Schilp DE, Hanak SE, Sorensen SM, Wettstein JG (2000) Effect of AMPA receptor modulators on hippocampal and cortical function. Eur J Pharmacol 394:85–90
Boulter J, Hollmann M, O’Shea-Greenfield A, Hartley M, Deneris, E, Maron, C, Heinemann S (1990) Molecular cloning and functional expression of glutamate receptor subunit genes. Science 249(4972):1033–1037
Boxall AR, Garthwaite J (1995) Synaptic excitation mediated by AMPA receptors in rat cerebellar slices is selectively enhanced by aniracetam and cyclothiazide Neurochem Res 20(5):605–609
Brene S, Messer C, Nestler EJ (1998) Expression of messenger RNAs encoding ionotropic glutamate receptors in rat brain: regulation by haloperidol. Neuroscience 84(3):813–823
Brorson JR, Li D, Suzuki T (2004) Selective expression of heteromeric AMPA receptors driven by flip-flop differences. J Neurosci 24(14):3461–3470
Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV (2004) The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology 46(1):10–22
Burnashev N, Monyer H, Seeburg PH, Sakmann B (1992) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8(1):189–198
Carroll RC, Beattie EC, von Zastrow M, Malenka RC (2001) Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev, Neurosci 2(5):315–324
Chapman AG, al-Zubaidy Z, Meldrum BS (1993) Aniracetam reverses the anticonvulsant action of NBQX and GYKI 52466 in DBA/2 mice. Eur J Pharmacol 231(2):301–303
Chazot PL (2004) The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem 11(3):389–396
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1(8):623–634
Cull-Candy S, Brickley S, Farrant M (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11(3):327–335
Cumin R, Bandle EF, Gamzu E, Haefely WE (1982) Effects of the novel compound aniracetam (Ro 13-5057) upon impaired learning and memory in rodents. Psychopharmacology (Berl) 78(2):104–111
Davis CM, Moskovitz B, Nguyen MA, Tran BB, Arai A, Lynch G, Granger R (1997) A profile of the behavioral changes produced by facilitation of AMPA-type glutamate receptors. Psychopharmacology (Berl) 133(2):161–167
De Sarro G, Siniscalchi A, Ferreri G, Gallelli L, De Sarro A (2000) NMDA and AMPA/kainate receptors are involved in the anticonvulsant activity of riluzole in DBA/2 mice. Eur J Pharmacol 10:408(1):25–34
Dicou E, Rangon CM, Guimiot F, Spedding M, Gressens P (2003) Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Brain Res 970(1–2):221–225
Doble A (1999) The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 81(3):163–221
Dudkin KN, Kruchinin VK, Chueva IV (1997) Synchronization processes in the mechanisms of short-term memory in monkeys: the involvement of cholinergic and glutaminergic cortical structures. Neurosci Behav Physiol 27:303–308
Eastwood SL, Burnet PW, Harrison PJ (1997) GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase–polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res 44(1):92–98
Fletcher EJ, Lodge D (1996) New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors. Pharmacol Ther 70(1):65–89
Frith C, Dolan R (1996) The role of the prefrontal cortex in higher cognitive functions. Cogn Brain Res 5:175–181
Frye GD, Fincher A (2000) Sustained ethanol inhibition of native AMPA receptors on medial septum/diagonal band (MS/DB) neurons. Br J Pharmacol 129(1):87–94
Gates M, Ogden A, Bleakman D (2001) Pharmacological effects of AMPA receptor potentiators LY392098 and LY404187 on rat neuronal AMPA receptors in vitro. Neuropharmacology 40(8):984–991
Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15(1):193–204
Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G (2001) A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol 21(5):484–487
Goldman-Rakic PS (1994) Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 6:348–357
Granger R, Stäubli U, Davis M, Perez Y, Nilsson L, Rogers GA, Lynch G (1993) A drug that facilitates glutamatergic transmission reduces exploratory activity and improves performance in a learning-dependent task. Synapse 15(4):326–329
Hampson RE, Rogers G, Lynch G, Deadwyler SA (1998a) Facilitative effects of the ampakine CX516 on short-term memory in rats: correlations with hippocampal neuronal activity. J Neurosci 18(7):2748–2763
Hampson RE, Rogers G, Lynch G, Deadwyler SA (1998b) Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci 18(7):2740–2747
Harrison NL, Simmonds MA (1985) Quantitative studies on some antagonists of N-methyl-D-aspartate in slices of rat cerebral cortex. Br J Pharmacol 84(2):381–391
Hashimoto K, Shimizu E, Iyo M (2004) Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev 45(2):104–114
Hayashi T, Umemori H, Mishina M, Yamamoto T (1999) The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature 7:397(6714):72–76
Hennegriff M, Arai A, Kessler M, Vanderklish P, Mutneja MS, Rogers G, Neve RL, Lynch G (1997) Stable expression of recombinant AMPA receptor subunits: binding affinities and effects of allosteric modulators. J Neurochem 68(6):2424–2434
Hollmann M, O’Shea-Greenfield A, Rogers SW, Heinemann S (1989) Cloning by functional expression of a member of the glutamate receptor family. Nature 342(6250):643–648
Hori M, Iemura R, Hara H, Sukamoto T, Ito K, Ohtaka H (1991) Potential nootropic agents, 4-alkoxy-2-(1-piperazinyl)quinazoline derivatives. Chem Pharm Bull (Tokyo) 39(2):367–371
Hume RI, Dingledine R, Heinemann SF (1991) Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253(5023):1028–1031
Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G (1997) Enhancement by an ampakine of memory encoding in humans. Exp Neurol 146(2):553–559
Ito I, Tanabe S, Kohda A, Sugiyama H (1990) Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam.J Physiol 424:533–543
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308
Johansen TH, Chaudhary A, Verdoorn TA (1995) Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol Pharmacol 48(5):946–955
Johnson SA, Luu NT, Herbst TA, Knapp R, Lutz D, Arai A, Rogers GA, Lynch G (1999) Synergistic interactions between ampakines and antipsychotic drugs. J Pharmacol Exp Ther 289(1):392–397
Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH (1990) A family of AMPA-selective glutamate receptors. Science 249(4968):556–560
Kessler M, Mutneja MS, Rogers G, Lynch G (1998) Regional preferences of AMPA receptor modulators determined through agonist binding autoradiography. Brain Res 783(1):121–126
Kimball JR, Johnson JA, Griffey KL, Ornstein PL, Zimmerman DM, Zarrinmayeh H, Schoepp DD, Tizzano JP (2000) The novel AMPA receptor potentiator, LY392098, enhances spatial learning and memory in a water maze. Abstr-Soc Neurosci 26:528.19
Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, Crites G, Malatynska E (2002) Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol 440(1):27–35
Koch HJ, Szecsey A, Haen E (2004) NMDA-antagonism (memantine): an alternative pharmacological therapeutic principle in Alzheimer’s and vascular dementia. Curr Pharm Des 10(3):253–259
Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S (2000) Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci 20:2166–2174
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB Jr, Vegso S, Heninger GR, Charney DS (1999a) Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacologia 145(2):193–204
Krystal JH, D’Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S (1999b) NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatr 7(3):125–143
Lambolez B, Ropert N, Perrais D, Rossier J, Hestrin S (1996) Correlation between kinetics and RNA splicing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci U S A 93(5):1797–1802
Larson J, Le T, Hall RA, Lynch G (1994) Effects of cyclothiazide on synaptic responses in slices of adult and neonate rat hippocampus. NeuroReport 5:389–392
Larson J, Quach CN, LeDuc BQ, Nguyen A, Rogers GA, Lynch G (1996) Effects of an AMPA receptor modulator on methamphetamine-induced hyperactivity in rats. Brain Res 738(2):353–356
Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM (2000) Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci 20(1):8–21
Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM (2003) Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther 307(1):297–305
Lee CR, Benfield P (1994) Aniracetam. An overview of its pharmacodynamic and pharmacokinetic properties, and a review of its therapeutic potential in senile cognitive disorders. Drugs Aging 4(3):257–273
Liddle PF (1992) Syndromes of schizophrenia on factor analysis. Br J Psychiatry 161:861
Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH (1994) Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 266(5191):1709–1713
Luby E, Cohen B, Rosenbaum G, Gottlieb J, Kelley R (1959) Study of a new schizophrenomimetic drug—Sernyl. AMA Arch Neurol Psych 81:363–369
Lynch G (2004) AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol 4(1):4–11
Lynch G, Baudry M (1984) The biochemistry of memory: a new and specific hypothesis.Science 8;224(4653):1057–1063
Lynch DR, Guttmann RP (2001) NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets 2(3):215–231
Lynch G, Kessler M, Rogers G, Ambros-Ingerson J, Granger R, Schehr RS (1996) Psychological effects of a drug that facilitates brain AMPA receptors. Int Clin Psychopharmacol 11(1):13–19
Lynch G, Granger R, Ambros-Ingerson J, Davis CM, Kessler M, Schehr R (1997) Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol 145(1):89–92
Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P (2002) An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology 43(1):1–10
Malenka RC (1991) The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol 5(2–4):289–295
Malenka RC (2003) Synaptic plasticity and AMPA receptor trafficking. Ann NY Acad Sci 1003:1–11
Malinow R (2003) AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond, B Biol Sci 358(1432):707–714
Marenco S, Egan MF, Goldberg TE, Knable MB, McClure RK, Winterer G, Weinberger DR (2002) Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: a case series. Schizophr Res 57(2–3):221–226
Martin JR, Cumin R, Aschwanden W, Moreau JL, Jenck F, Haefely WE (1992) Aniracetam improves radial maze performance in rats. NeuroReport 3(1):81–83
Meador-Woodruff JH, Healy DJ (2000) Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev 31(2–3):288–294
Miu P, Jarvie KR, Radhakrishnan V, Gates MR, Ogden A, Ornstein PL, Zarrinmayeh H, Ho K, Peters D, Grabell J, Gupta A, Zimmerman DM, Bleakman D (2001) Novel AMPA receptor potentiators LY392098 and LY404187: effects on recombinant human AMPA receptors in vitro. Neuropharmacology 40(8):976–983
Mohler H, Fritschy JM, Rudolph U (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300(1):2–8
Monyer H, Seeburg PH, Wisden W (1991) Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 6(5):799–810
Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP (1994) A molecular determinant for submillisecond desensitization in glutamate receptors.Science 266(5187):1059–1062
Murray TK, Whalley K, Robinson CS, Ward MA, Hicks CA, Lodge D, Vandergriff JL, Baumbarger P, Siuda E, Gates M, Ogden AM, Skolnick P, Zimmerman DM, Nisenbaum ES, Bleakman D, O’Neill MJ (2003) LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson’s disease. J Pharmacol Exp Ther 306(2):752–762
Nagarajan N, Quast C, Boxall AR, Shahid M, Rosenmund C (2001) Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546. Neuropharmacology 41(6):650–663
Nakamura K, Kurasawa M (2000) Serotonergic mechanisms involved in the attentional and vigilance task performance of rats and the palliative action of aniracetam. Naunyn-Schmiedeberg’s Arch Pharmacol 361(5):521–528
Nakamura K (2002) Aniracetam: its novel therapeutic potential in cerebral dysfunctional disorders based on recent pharmacological discoveries. CNS Drug Rev 8(1):70–89
Nakamura K, Kurasawa M (2001) Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism. Eur J Pharmacol 420(1):33–43
Nakamura K, Tanaka Y (2001) Antidepressant-like effects of aniracetam in aged rats and its mode of action. Psychopharmacology (Berl) 158(2):205–212
Nakamura K, Kurasawa M, Tanaka Y (1998a) Apomorphine-induced hypoattention in rats and reversal of the choice performance impairment by aniracetam. Eur J Pharmacol 342:127–138
Nakamura K, Kurasawa M, Tanaka Y (1998b) Scopolamine model of delirium in rats and reversal of the performance impairment by aniracetam. Drug Dev Res 43:85–97
Nakamura K, Kurasawa M, Shirane M (2000) Impulsivity and AMPA receptors: aniracetam ameliorates impulsive behavior induced by a blockade of AMPA receptors in rats. Brain Res 862(1–2):266–269
Ogasawara T, Itoh Y, Tamura M, Mushiroi T, Ukai Y, Kise M, Kimura K (1999) Involvement of cholinergic and GABAergic systems in the reversal of memory disruption by NS-105, a cognition enhancer. Pharmacol Biochem Behav 64(1):41–52
Ohno M, Yamamoto T, Kitajima I, Ueki S (1990) WEB 1881 FU ameliorates impairment of working memory induced by scopolamine and cerebral ischemia in the three-panel runway task. Jpn J Pharmacol 54(1):53–60
Olney JW, Newcomer JW, Farber NB (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33(6):523–533
O’Neill MJ, Murray TK, Whalley K, Ward MA, Hicks CA, Woodhouse S, Osborne DJ, Skolnick P (2004) Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. Eur J Pharmacol 486(2):163–174
Park Y, Jang CG, Yang KH, Loh HH, Ma T, Ho IK (2003) Regional specific increases of [3H]AMPA binding and mRNA expression of AMPA receptors in the brain of mu-opioid receptor knockout mice. Brain Res Mol Brain Res 113(1–2):116–123
Partin KM, Patneau DK, Mayer ML (1994) Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol 46(1):129–138
Petralia RS, Wenthold RJ (1992) Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol 318:329–354
Pontecorvo MJ, Evans HL (1985) Effects of aniracetam on delayed matching-to-sample performance of monkeys and pigeons. Pharmacol Biochem Behav 22(5):745–752
Quirk JC, Nisenbaum ES (2002) LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Rev 8(3):255–282
Quirk JC, Nisenbaum ES (2003) Multiple molecular determinants for allosteric modulation of alternatively spliced AMPA receptors. J Neurosci 2623(34):10953–10962
Rao Y, Xiao P, Xu S (2001) Effects of intrahippocampal aniracetam treatment on Y-maze avoidance learning performance and behavioral long-term potentiation in dentate gyrus in rat. Neurosci Lett 298(3):183–186
Rogan MT, Stäubli UV, LeDoux JE (1997) AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci 17(15):5928–5935
Romanides AJ, Duffy P, Kalivas PW (1999) Glutamatergic and dopaminergic afferents to the prefrontal cortex regulate spatial working memory in rats. Neuroscience 92:97–106
Rosa ML, Guimaraes FS, Pearson RC, Del Bel EA (2002) Effects of single or repeated restraint stress on GluR1 and GluR2 flip and flop mRNA expression in the hippocampal formation. Brain Res Bull 59(2):117–124
Satoh M, Ishihara K, Iwama T, Takagi H (1986) Aniracetam augments, and midazolam inhibits, the long-term potentiation in guinea-pig hippocampal slices. Neurosci Lett 68(2):216–220
Sekiguchi M, Yamada K, Jin J, Hachitanda M, Murata Y, Namura S, Kamichi S, Kimura I, Wada K (2001) The AMPA receptor allosteric potentiator PEPA ameliorates post-ischemic memory impairment. NeuroReport 12(13):2947–2950
Shors TJ, Servatius RJ, Thompson RF, Rogers G, Lynch G (1995) Enhanced glutamatergic neurotransmission facilitates classical conditioning in the freely moving rat. Neurosci Lett 186(2–3):153–156
Siegel GJ, Chauhan NB (2000) Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev 33(2–3):199–227
Smith PF (2003) Therapeutic N-methyl-D-aspartate receptor antagonists: will reality meet expectation? Curr Opin Investig Drugs 4(7):826–832
Sommer B, Seeburg PH (1992) Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci 13(7):291–296
Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH (1990) Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science 249(4976):1580–1585
Stäubli U, Ambros-Ingerson J, Lynch G (1992) Receptor changes and LTP: an analysis using aniracetam, a drug that reversibly modifies glutamate (AMPA) receptors. Hippocampus 2(1):49–57
Stäubli U, Rogers G, Lynch G (1994) Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci U S A 91(2):777–781
Stein E, Cox JA, Seeburg PH, Verdoorn TA (1992) Complex pharmacological properties of recombinant alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subtypes. Mol Pharmacol 42(5):864–871
Stine CD, Lu W, Wolf ME (2001) Expression of AMPA receptor flip and flop mRNAs in the nucleus accumbens and prefrontal cortex after neonatal ventral hippocampal lesions. Neuropsychopharmacology 24(3):253–266
Suzuki T, Tsuzuki K, Kameyama K, Kwak S (2003) Recent advances in the study of AMPA receptors. Nippon Yakurigaku Zasshi 122(6):515–526
Tallaksen-Greene SJ, Albin RL (1996) Splice variants of glutamate receptor subunits 2 and 3 in striatal projection neurons. Neuroscience 75(4):1057–1064
Teyler TJ, Cavus I, Coussens C, DiScenna P, Grover L, Lee YP, Little Z (1994) Multideterminant role of calcium in hippocampal synaptic plasticity. Hippocampus 4(6):623–634
Thompson DM, Guidotti A, DiBella M, Costa E (1995) 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proc Natl Acad Sci U S A 92(17):7667–7671
Tizzano JP, Kimball JR, Johnson JA, Griffey KL (2000) The novel AMPA receptor potentiator, LY392098, reverses pharmacologically and age-induced memory deficits in rats. Abstr-Soc Neurosci 26:528.20
Togashi H, Nakamura K, Matsumoto M, Ueno K, Ohashi S, Saito H, Yoshioka M (2002) Aniracetam enhances glutamatergic transmission in the prefrontal cortex of stroke-prone spontaneously hypertensive rats. Neurosci Lett 320(3):109–112
Tomiyama M, Palacios JM, Cortes R, Mengod G (1999) Flip and flop variants of AMPA receptor subunits in the human cerebellum: implication for the selective vulnerability of Purkinje cells. Synapse 31(2):163–167
Vandergriff J, Huff K, Bond A, Lodge D (2001) Potentiation of responses to AMPA on central neurones by LY392098 and LY404187 in vivo. Neuropharmacology 40(8):1003–1009
Vanover KE (1997) Effects of AMPA receptor positive modulators on amphetamine- and dizocilpine-induced locomotion. Eur J Pharmacol 332(2):115–119
Weinberger D, Berman KF (1996) Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond, B Biol Sci 351:1495–1503
Whiting PJ (2003) GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today 8(10):445–450
Wisden W, Seeburg PH (1993) Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol 3(3):291–298
Yamada K (1998) Modulating excitatory synaptic neurotransmission: potential treatment for neurological disease? Neurobiol Dis 5:67–80
Zajaczkowski W, Danysz W (1997) Effects of D-cycloserine and aniracetam on spatial learning in rats with entorhinal cortex lesions. Pharmacol Biochem Behav 56(1):21–29
Zavitsanou K, Ward PB, Huang XF (2002) Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology 27(5):826–833
Zeng L, Lu L, Muller M, Gouaux E, Zhou MM (2002) Structure-based functional design of chemical ligands for AMPA-subtype glutamate receptors. J Mol Neurosci 19(1–2):113–116
Zeng L, Chen CH, Muller M, Zhou MM (2003) Structure-based rational design of chemical ligands for AMPA-subtype glutamate receptors. J Mol Neurosci 20(3):345–348
Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A (1995) 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. J Pharmacol Exp Ther 272(1):300–309
Acknowledgements
The author would like to thank Beth Borowsky, Kathleen McMonagle-Strucko, and Sam Kongsamut for scientific and technical discussion during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Black, M.D. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology 179, 154–163 (2005). https://doi.org/10.1007/s00213-004-2065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-2065-6