Abstract
Rationale
The α7 subtype of the nicotinic receptor plays an important role in auditory sensory gating. Schizophrenics show deficient sensory gating and abnormalities in the number and regulation of nicotinic receptors. Prepulse inhibition (PPI) deficits exhibited by isolation-reared rats are thought to model the sensorimotor gating deficits seen in schizophrenia.
Objective
To examine the role of nicotinic α7 receptors in the isolation-rearing rat model, we tested whether the selective α7 receptor agonist (R)-N-(1-Azabicyclo[2.2.2]oct-3-yl)(5-(2-pyridyl)thiophene-2-carboxamide) (compound A) could reverse isolation-rearing-induced PPI deficits, and investigated α7 receptor RNA expression in the hippocampus, prefrontal cortex, cerebellum, nucleus accumbens and thalamus, and α7 receptor protein expression in the hippocampus of isolation- and group-reared rats.
Method
Rats reared in isolation or groups of five from weaning were tested in the PPI paradigm under conditions of variable inter-stimulus interval (ISI) (pulse=110 dB/50 ms; prepulse=75 dB/30 ms; ISI=30, 100 and 300 ms) 30 min following administration of compound A (3.2–10 mg/kg i.p.). α7 Receptor expression was measured by TaqMan RT-PCR (total RNA) and autoradiography (protein).
Results
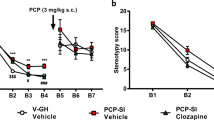
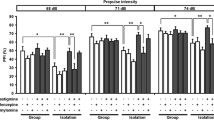
Isolation-rearing-induced PPI deficits were attenuated by both doses of compound A at 100-ms ISI and by 10 mg/kg at 300-ms ISI. Expression of α7 receptor RNA and protein was unaltered in isolation-reared rats.
Conclusion
Although altered α7 receptor expression may not underlie the phenotype of isolation-reared rats, the activation of these receptors may be of benefit in re-establishing efficient gating function.

Similar content being viewed by others
References
Bakshi VP, Swerdlow NR, Braff DL, Geyer MA (1998) Reversal of isolation rearing-induced deficits in prepulse inhibition by Seroquel and olanzapine. Biol Psychiatry 43:436–445
Bolino F, DiMichele V, DiCocco L, Manna V, Daneluzzo E, Casacchia M (1994) Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenics. Biol Psychiatry 36:670–679
Bond BC, Virley DJ, Cairns NJ, Hunter AJ, Moore GB, Moss SJ, Mudg AW, Walsh FS, Jazin E, Preece P (2002) The quantification of gene expression in an animal model of brain ischemia using TaqMan real-time RT-PCR. Brain Res Mol Brain Res 106(1–2):101–116
Braff DL (1989) Sensory input deficits and negative symptoms in schizophrenic patients. Am J Psychiatr 146:1006–1011
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry 47:181–188
Braff DL, Grillon C, Geyer MA (1992) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 49:206–215
Braff DL, Swerdlow NR, Geyer MA (1995) Gating and habituation deficits in the schizophrenia disorders. Clin Neurosci 3:131–139
Brenner CA, Edwards CR, Carrol CA, Kieffaber PD, Hetrick WP (2004) P50 and acoustic startle gating are not related in healthy participants. Psychophysiology 41:702–708
Cilia J, Reavill C, Hagan JJ, Jones DNC (2001) Long-term evaluation of isolation-reared prepulse inhibition deficits in rats. Psychopharmacology 156:327–337
Cilia J, Hatcher PD, Reavill C, Jones DNC (2005) Long-term evaluation of isolation-reared prepulse inhibition deficits in rats: an update. Psychopharmacology, available on-line; DOI: 10.1007/s00213-004-2139-5
Davies ARL, Hardick DJ, Blagbrough IS, Potter BVL, Wolstenholme AJ, Wonnacott S (1999) Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling α7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology 38:679–690
De Filippi G, Broad LM, Zwart R, Baldwinson T, Felthouse C, McPhie G, Pearson K, Craig P, Broadmore R, Bell F, Cases M, Keenan M, Baker SR, Boot J, Sher E (2002) Pharmacological characterisation and in vitro physiological effects of new 7 nicotinic acetylcholine receptor agonists. Society for Neuroscience, Washington, DC, program no. 137.5
Freedman R, Hal M, Adler LE, Leonard S (1995) Evidence in post-mortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156(2–3):117–154
Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther 294:1112–1119
Hoffman HS, Ison JR (1980) Reflex modification in the domain of startle. I. Some empirical findings and their implication for how the nervous system processes sensory input. Psychol Rev 87:175–189
Leonard S, Gault J, Adams C, Breese CR, Rollins Y, Adler LE, Olincy A, Freedman R (1998) Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci 12:1–7
Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebin C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R (2002) Association of promoter variants in the a7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 59:1085–1096
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149
Luntz-Leybman V, Bickford PC, Freedman R (1992) Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res 587:130–136
Martin LF, Kem WR, Freedman R (2004) Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology 174:54–64
McEvoy JP, Allen TB (2002) The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette's syndrome. Curr Drug Target CNS Neurol Disord 1:433–442
Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN (2000) The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods 98(1):9–20
Mugnaini M, Tessari M, Tarter G, Merlo Pich E, Chiamulera C, Bunnemann B (2002) Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of α7 or α6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur J Neurosci 16:1633–1646
Nakato K, Morita T, Wanibuchi F, Yamaguchi T (1997) Antipsychotics restored but antidepressants and anxiolytics did not restore prepulse inhibition (PPI) deficits in isolation-reared rats. Soc Neurosci Abstr 23:1855
O'Neill MJ, Moore NA, McKinzie DL, Keenan M, Wishart G, Shannon HE, Murray TK, O'Neill MF, Tree B, Iyengar S, Hart J, Shaw D, Simmons RMA, Kalar AB, Miles C, Baker SR, Sher E, Tricklebank MD (2002) Behavioural profiling of two potent alpha-7 nicotinic acetylcholine receptor agonists. Society for Neuroscience, Washington, DC, program no. 137.6
O'Neill HC, Rieger K, Kem WR, Stevens KE (2003) DMXB, an α7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology 169:332–339
Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP (2000) Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry 47:662–669
Simosky JK, Stevens KE, Freedman R (2002) Nicotinic agonists and psychosis. Curr Drug Target CNS Neurol Disord 1:149–162
Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM (1996) Genetic correlation of inhibitory gating of hippocampal auditory evoked response and α-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology 15(2):152–162
Suemaru K, Yasuda K, Umeda K, Araki H, Shibata K, Choshi T, Hibino S, Gomita Y (2004) Nicotine blocks apomorphine-induced disruption of prepulse inhibition of the acoustic startle in rats: possible involvement of central nicotinic α7 receptors. Br J Pharmacol 142:843–850
Swerdlow NR, Braff DL, Taaid N, Geyer MA (1994) Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51:139–153
Swerdlow NR, Braff DL, Geyer MA (2000) Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 11:185–204
Weiss IC, Feldon J (2001) Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology 156(2–3):305–326
Whiteaker P, Davies ARL, Marks MJ, Blagbrough IS, Potter BVL, Wolstenholme AJ, Collins AC, Wonnacott S (1999) An autoradiographic study of the distribution of binding sites for the novel α7-selective nicotinic radioligand [3H]methyllycaconitine in the mouse brain. Eur J Neurosci 11:2689–2696
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cilia, J., Cluderay, J.E., Robbins, M.J. et al. Reversal of isolation-rearing-induced PPI deficits by an α7 nicotinic receptor agonist. Psychopharmacology 182, 214–219 (2005). https://doi.org/10.1007/s00213-005-0069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0069-5