Abstract
Rationale
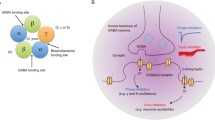
Cortical γ-aminobutyric acid (GABA)ergic neurons contribute to the orchestration of pyramidal neuron population firing as follows: (1) by releasing GABA on GABAA and GABAB receptors, (2) by releasing reelin in the proximity of integrin receptors located on cortical pyramidal neuron dendritic spines, and (3) through reelin contributing to the regulation of dendritic spine plasticity by modulating dendritic resident mRNA translation. In schizophrenia (SZ) and bipolar (BP) postmortem brains, the downregulation of mRNAs encoding glutamic acid decarboxylase 67 (GAD67) and reelin decreases the cognate proteins coexpressed in prefrontal cortex (PFC) GABAergic neurons. This finding has been replicated in several laboratories. Such downregulation suggests that the neuropil hypoplasticity found in the PFC of SZ and BP disorder patients may depend on a downregulation of GABAergic function, which is associated with a decrease in reelin secretion from GABAergic neuron axon terminals on dendrites, somata, or axon initial segments of pyramidal neurons. Indirectly, this GABAergic neuron downregulation may play a key role in the expression of positive and negative symptoms of SZ and BP disorders.
Objectives
The above described GABAergic dysfunction may be addressed by pharmacological interventions to treat SZ and BP disorders using specific benzodiazepines (BZs), which are devoid of intrinsic activity at GABAA receptors including α1 subunits but that act as full positive allosteric modulators of GABA action at GABAA receptors containing α2, α3, or α5 subunits. These drugs are expected to enhance GABAergic signal transduction without eliciting sedation, amnesia, and tolerance or dependence liabilities.
Results and conclusions
BZs, such as diazepam, although they are efficient in equilibrating GABAA receptor signal transduction in a manner beneficial in the treatment of positive and negative symptoms of SZ, may not be ideal drugs, because by mediating a full positive allosteric modulation of GABAA receptors containing the α1 subunit, they contribute to sedation and to the development of tolerance after even a brief period of treatment. In contrast, other BZ-binding site ligands, such as 6-(2bromophenyl)-8-fluoro-4H-imidazo [1,5-a][1,4] benzodiazepine-3-carboxamide (imidazenil), which fail to allosterically and positively modulate the action of GABA at GABAA receptors with α1 subunits but that selectively allosterically modulate cortical GABAA receptors containing α5 subunits, contribute to the anxiolytic, antipanic, and anticonvulsant actions of these ligands without producing sedation, amnesia, or tolerance. Strong support for the use of imidazenil in psychosis emerges from experiments with reeler mice or with methionine-treated mice, which express a pronounced reelin and GAD67 downregulation that is also operative in SZ and BP disorders. In mice that model SZ symptoms, imidazenil increases signal transduction at GABAA receptors containing α5 subunits and contributes to the reduction of behavioral deficits without producing sedation or tolerance liability. Hence, we suggest that imidazenil may be considered a prototype for a new generation of positive allosteric modulators of GABAA receptors, which, either alone or in combination with neuroleptics, should be evaluated in GABAergic dysfunction operative in the treatment of SZ and BP disorders with psychosis.






Similar content being viewed by others
References
Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M et al (2005) Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenia patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 134:60–66
Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE Jr, Jones EG (1995a) GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex 6:550–560
Akbarian S, Kim JJ, Potkin G, Hagman JO, Tafazzoli A, Bunney WE Jr, Jones EG (1995b) Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 552:258–266
Auta J, Giusti P, Guidotti A, Costa E (1994) Imidazenil, a partial positive allosteric modulator of GABAA receptors, exhibits low tolerance and dependence liabilities in the rat. J Pharmacol Exp Ther 270:1262–1269
Auta J, Fraust WB, Lambert P, Guidotti A, Costa E, Moerschbaecher JM (1995) Comparison of the effects of full and partial allosteric modulators of GABAA receptors on complex behavioral processes in monkeys. Behav Pharmacol 6:323–332
Auta J, Guidotti A, Costa E (2000) Imidazenil prevention of alprazolam-induced acquisition in Patas monkeys is devoid of tolerance. Proc Natl Acad Sci U S A 97:2314–2319
Auta J, Costa E, Davis J, Guidotti A (2004) Imidazenil: a potent and safe protective agent against diisopropyl fluorophosphate toxicity. Neuropharmacology 46:397–403
Barnard EA (2001) The molecular architecture of GABAA receptors. In: Möhler H (ed) Handbook of experimental pharmacology, vol 150. Springer, Berlin Heidelberg New York, pp 79–99
Benes FM (2000) Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev 31:251–269
Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27
Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP (1992) Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci 12:924–929
Benes FM, Khan Y, Vincent SL, Wickramasinghe R (1996a) Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse 22:338–349
Benes FM, Vincent SL, Marie A, Khan Y (1996b) Upregulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience 75:1021–1031
Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT (2004) Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry 161:742–744
Busto U, Kaplan HL, Zawertailo L, Sellers EM (1994) Pharmacologic effects and abuse liability of bretazenil, diazepam, and alprazolam in humans. Clin Pharmacol Ther 55:451–463
Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML (2001) Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 41:237–260
Carpenter WT Jr, Buchanan RW, Kirkpatric B, Breier AF (1999) Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry 156:299–303
Carter CS, Botvinick MM, Cohen JD (1999) The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 10:49–57
Costa E, Guidotti A (1996) Benzodiazepines on trial: a research strategy for their rehabilitation. TIPS 17:192–200
Costa E, Auta J, Guidotti A (2001a) Tolerance and dependence to ligands of the benzodiazepine recognition sites expressed by GABAA receptors. In: Möhler E (ed) Handbook of experimental pharmacology, vol 150. Pharmacology of GABA and glycine neurotransmission. Springer, Berlin Heidelberg New York, pp 227–250
Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C (2001b) Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis 8:723–742
Costa E, Auta J, Grayson DR, Matsumoto K, Pappas DG, Zhang X, Guidotti A (2002a) GABAA receptors and benzodiazepines: a role for dendritic resident subunit mRNAs. Neuropharmacology 43:925–937
Costa E, Davis J, Pesold C, Tueting P, Guidotti A (2002b) The heterozygote reeler mouse as a model for the development of a new generation of antipsychotics. Curr Opin Pharmacol 2:56–62
Costa E, Grayson DR, Mitchell CP, Tremolizzo L, Veldic M, Guidotti A (2003) GABAergic cortical neuron chromatin as a putative target to treat schizophrenia vulnerability. Crit Rev Neurobiol 15:121–142
Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L, Veldic M (2004) A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol 16:1–24
Crestani F, Löw K, Deist R, Mandelli M, Möhler H, Rudolph U (2001) Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol 59:442–445
Crick FH, Koch C (2003) A framework for consciousness. Nat Neurosci 6:119–126
Cruz DA, Eggan SM, Lewis DA (2003) Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 465:385–400
Dean B, Hussain T, Hayes W, Scarr E, Kitsoulis S, Hill C, Opeskin K (1999) Changes in serotonin2A and GABA (A) receptors in schizophrenia: studies on the human dorsolateral prefrontal cortex. J Neurochem 72:1593–1599
Dean B, Pavey G, McLeod M, Opeskin K, Keks N, Copolov D (2001) A change in the density of [(3)H]flumazenil, but not [(3)H]muscimol binding, in Brodmann’s Area 9 from subjects with bipolar disorder. J Affect Disord 66:147–158
DeFilipe J, Farinas I (1992) The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 39:563–607
Delini-Stula A, Berdah-Tordjman D, Neumann N (1992) Partial benzodiazepine antagonists in schizophrenia: expectations and present clinical findings. Clin Neuropharmacol 15:405A–406A
Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, Guidotti A (2003) The reelin–integrin interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci U S A 100:5479–5484
Eastwood SL, Harrison PJ (2003) Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry 8:821–831
Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK (2004) Specific GABAA circuits for visual cortical plasticity. Science 303:1681–1683
Fatemi SH, Earle JA, McMenomy T (2000) Reduction in reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5:654–663
Fritschy JM, Möhler H (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194
Fritschy JM, Weinman O, Wenzel A, Benke D (1998) Synapse-specific localization of NMDA and GABA (A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol 390:194–210
Giusti P, Guidotti A, Danysz W, Auta J, Costa E (1990) Neuropharmacological evidence for an interaction between the GABA uptake inhibitor CI-966 and anxiolytic benzodiazepines. Drug Dev Rev 21:217–225
Giusti P, Ducic I, Puia G, Arban R, Walser A, Guidotti A, Costa E (1993) Imidazenil: a new partial positive allosteric modulator of γ-aminobutyric acid (GABA) action at GABAA receptors. J Pharmacol Exp Ther 266:1018–1028
Glantz LA, Lewis DA (2001) Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry 58:203
Guidotti A, Gale K, Suria A, Toffano G (1979) Biochemical evidence for two classes of GABA receptors in rat brain. Brain Res 172:566–571
Guidotti A, Auta J, Davis JM, Dwivedi Y, Gerevini VD, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov DP, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069
Halasy K, Buhl EH, Lorinczi Z, Tamas G, Somogyi P (1996) Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus 6:306–329
Hanada S, Mita T, Nishino N (1987) [3H] muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sci 40:259–266
Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM (2002) Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry 59:521–529
Hensch TK, Stryker MP (2004) Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science 303:1619–1621
Impagnatiello F, Pesold C, Longone P, Caruncho HJ, Fritschy JM, Costa E, Guidotti A (1996) Modifications of γ aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol 49:822–831
Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H, Pisu M, Uzunov D, Smalheiser N, Davis J, Pandey G, Pappas G, Tueting P, Sharma R, Costa E (1998) A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A 95:15718–15723
Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T (2004) Immunohistochemical and immunoblot study of GABA(A) alpha1 and beta2/3 subunits in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci Res 50:77–84
Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E (2001) Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci U S A 98:3483–3488
Jakab RL, Goldman-Rakic PS (2000) Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol 417(3):337–348
Jones EG (1993) GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 3:361–372
Jones EW (2000) Microcolumns in the cerebral cortex. Proc Natl Acad Sci U S A 97:5019–5021
Knable MB, Barci BM, Webster MJ, Woodruff JM, Torrey EF (2004) Molecular abnormalities in the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 9:609–620
Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA (2003) Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71:67–80
Lees AJ, Clarke CR, Harrison MJ (1977) Hallucinations after withdrawal of baclofen. Lancet 16:858
Levitan FS, Schofield PR, Burt DR, Rhee LM, Wisden W, Kohler M, Fujita N, Rodriguez HF, Stephenson A, Darlinson MG, Barnard EA, Seeburg PH (1988) Structural and functional basis for GABAA receptor heterogeneity. Nature 335:76–79
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334
Lewis DA, Volk DW, Hashimoto T (2004) Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology 174:143–150
Longone P, Impagnatiello F, Guidotti A, Costa E (1996) Reversible modifications of GABAA receptor subunit mRNA expression during tolerance to diazepam-induced cognition dysfunction. Neuropharmacology 35:1465–1473
McBain CJ, Fisahn A (2001) Interneurons unbound. Nat Rev Neurosci 2:11–23
Miles R, Toth K, Gulyas AI, Hajos N, Freund GF (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16:815–823
Möhler H, Crestani F, Rudolph U (2001) GABA(A)-receptor subtypes: a new pharmacology. Curr Opin Pharmacol 1:22–25
Mountcastle VB (1998) Perceptual Neuroscience. The cerebral cortex. Harvard University Press, Cambridge, MA
Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G (2004) Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 41:71–84
Nusser Z, Sieghart W, Benke D, Fritschy JH, Somogyi P (1996) Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A 93:11939–11944
Nyiri G, Freund TF, Somogyi P (2001) Input-dependent synaptic targeting of alpha (2)-subunit-containing GABA (A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13:428–442
Perlstein WM, Carter CS, Noll DC (2001) Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenics. Am J Psychiatry 158:1105–1113
Peters A (1984) Chandelier cells. In: Jones EG, Peters A (eds) Cerebral cortex, vol 1. Plenum Press, NY, pp 361–380
Primus RJ, Gallager DW (1992) GABA receptor subunit mRNA levels are differentially influenced by chronic FG7142 and diazepam exposure. Eur J Pharmacol 226:21–28
Rao SG, Williams GV, Goldman-Rakic PS (1999) Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol 81:1903–1916
Rao SG, Williams GV, Goldman-Rakic PS (2000) Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci 20:485–494
Rice DS, Curran T (2001) Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 24:1005–1039
Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A (2002) In Patas monkey GAD67 and reelin mRNA coexpression varies in a manner dependent of layer and cortical area. J Comp Neurol 451:279–288
Rosoklija G, Toomayan G, Ellis S, Keilp J, Mann JJ, Latov N, Hays AP, Andrew AJ (2000) Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry 57:349–356
Sawaguchi T, Iba M (2001) Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. J Neurophysiol 86:2041–2053
Sawaguchi T, Matsumura M, Kubota K (1989) Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res 75:457–469
Selemon LD, Goldman-Rakic PS (1999) The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45:17–25
Sieghart W (1995) Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharm Rev 47:181–234
Skausig OB, Korsgaard S (1977) Hallucinations and baclofen. Lancet 1:158
Soghomonian JJ, Martin DL (1998) Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 19:500–505
Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW (2004) Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci 101:17288–17293
Stewart O, Schuman EM (2003) Compartmentalized synthesis and degradation of proteins in neurons. Neuron 9:347–359
Thompson DM, Guidotti A, Costa E (1995) Imidazenil, a new anxiolytic and anticonvulsant drug, attenuates a benzodiazepine-induced cognition deficit in monkeys. J Pharmacol Exp Ther 273:1307–1312
Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A (2002) An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A 99:17095–17100
Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A (2005) Valproate corrects a methionine-induced schizophrenia neuropathology in mouse. Biol Psychiatry 57:500–509
Veldic M, Caruncho H, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E (2004) DNA methyltransferase-1 (DNMT1) is selectively overexpressed in telencephalic cortical GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A 101:348–353
Veldic M, Guidotti A, Maloku E, Davis JM, Costa E (2005) In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A 102:2152–2157
Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA (2000) Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 57:237–245
Volk D, Austin M, Pierri J, Sampson A, Lewis D (2001) GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry 158:256–265
Wassef A, Baker J, Kochan LD (2003) GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 23:601–640
Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277:39944–39952
Williams GV, Rao SG, Goldman-Rakic PS (2002) The physiological role of 5-HT2A receptors in working memory. J Neurosci 1(22):2843–2854
Willins DL, Deutch AY, Roth BL (1997) Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27(1):79–82
Wolkowitz DM, Pickar D (1991) Benzodiazepines in the treatment of schizophrenia: a review and reappraisal. Am J Psychiatry 148:714–726
Woo TU, Whitehead RE, Melchitzky DS, Lewis DA (1998) A subclass of prefrontal γ-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A 95:5341–5346
Woo TU, Walsh JP, Benes FM (2004) Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-d-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry 61:649–657
Wyatt RJ, Benedict A, Davis J (1971) Biochemical and sleep studies of schizophrenia: a review of the literature 1960–1970. Schizophr Bull 4:10–44
Acknowledgements
This work is supported by RO1MH62188 to A.G., RO1MH62090 to E.C., and RO1MH6262A to D.R.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to Professor Joseph A. Flaherty on the occasion of his promotion to Dean of the UIC College of Medicine.
Rights and permissions
About this article
Cite this article
Guidotti, A., Auta, J., Davis, J.M. et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology 180, 191–205 (2005). https://doi.org/10.1007/s00213-005-2212-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2212-8