Abstract
Rationale and objectives
Lesion studies have shown that the tuberomammillary nucleus (TM) exerts inhibitory effects on the brain reward system. To determine whether histamine from the TM is involved in that reward inhibitory function, we assessed the stimulant and rewarding effects of cocaine in knockout mice lacking histidine decarboxylase (HDC KO mice), the histamine-synthesizing enzyme. If histamine actually plays an inhibitory role in reward, then it would be expected that mice lacking histamine would be more sensitive to the behavioral effects of cocaine.
Materials and methods
The first experiment characterized spontaneous locomotion and cocaine-induced hyperactivity (0, 8, and 16 mg/kg, i.p.) in wild-type and HDC KO mice. The rewarding effects of cocaine were investigated in a second experiment with the place-conditioning technique.
Results
The first experiment demonstrated that histidine decarboxylase mice showed reduced exploratory behaviors but normal habituation to the test chambers. After habituation to the test chambers, HDC KO mice were slightly, but significantly, less stimulated by cocaine than control mice. This finding was replicated in the second experiment, when cocaine-induced activity was monitored with the place-conditioning apparatus. Furthermore, a significant place preference was present in both genotypes for 8 and 16 mg/kg cocaine, but not for 2 and 4 mg/kg.
Conclusions
Our data confirm previous results demonstrating that HDC KO mice show reduced exploratory behaviors. However, contrary to the hypothesis that histamine plays an inhibitory role in reward, histamine-deficient mice were not more responsive to the psychostimulant effects of cocaine.
Similar content being viewed by others
Introduction
Neuronal histamine is produced by neurons exclusively located in the tuberomammillary nucleus (TM) of the posterior hypothalamus that innervate most areas of the brain. Histamine is synthesized from l-histidine by the enzyme histidine decarboxylase (HDC). To date, three classes of histamine receptors have been identified in the brain: the H1, H2, and H3 receptors. H1 and H2 receptors are mainly located post-synaptically and their activation leads to excitatory and facilitatory effects. In contrast, histamine H3 receptors are presynaptic inhibitory receptors. They are located on histaminergic terminals (H3 autoreceptors) and their activation produces an inhibition of histamine release (Arrang et al. 1983). H3 receptors are also present on non-histaminergic neurons (H3 heteroreceptors) and can inhibit the release of other neurotransmitters, such as glutamate, GABA, noradrenaline, serotonin, acetylcholine, and dopamine (Leurs et al. 2005).
In the brain, the histaminergic system is involved in many physiological and behavioral functions. Histamine is implicated in circadian rhythms and sleep, antinociception, water and food consumption, anxiety and learning (Brown et al. 2001; Haas and Panula 2003). Although conflicting data have been published, several studies have also demonstrated that histamine plays a role in reward and in addiction. For example, unilateral electrolytic or ibotenic acid lesions of the TM specifically increased lateral hypothalamic self-stimulation on the side ipsilateral to the lesion (Wagner et al. 1993a,b). Consistently, injection of histamine or histidine into the lateral hypothalamus specifically suppressed self-stimulation on the side ipsilateral to the injection (Cohn et al. 1973). Based on these findings, the TM of the hypothalamus was hypothesized to function as a reward-inhibiting substrate. According to that hypothesis, the reported increase in hypothalamic self-stimulation after lesions of the TM is interpreted as a “disinhibition” of reward processes (Huston et al. 1997; Zimmermann et al. 1999).
The histaminergic system was proposed to account for the inhibitory effects on reward produced by lesions of the TM (Huston et al. 1997; Dere et al. 2003). However, lesions of the TM may not only lead to neuronal histamine depletion, but also to the depletion of the transmitter systems co-localized in the TM or even co-released by histaminergic neurons (Brown et al. 2001). Most of the studies, having found that the histaminergic system inhibits reward, were performed with first generation H1 blockers. These reports found that inhibitions of the histaminergic system with H1 antagonists potentiate the rewarding properties of addictive drugs. Some H1 blockers were even reported to induce reinforcing effects alone. For example, the H1 antagonists tripelennamine, chlorpheniramine, and diphenhydramine maintained self-administration behaviors when substituted for cocaine in monkeys (Bergman and Spealman 1986; Beardsley and Balster 1992; Sannerud et al. 1995). Moreover, intraperitoneal injections of tripelennamine or chlorpheniramine yielded conditioned place preference in rats (Suzuki et al. 1999). Another study did not find a conditioned place preference after subcutaneous injections of tripelennamine alone, but reported that this H1 antagonist revealed conditioned place preferences induced by subthreshold doses of pentazocine or morphine (Suzuki et al. 1991). Consistently, subcutaneous injections of chlorpheniramine also failed to produce a conditioned place preference when administered alone, but revealed the reinforcing effects of low inactive doses of dihydrocodeine or methamphetamine (Suzuki et al. 1990; Masukawa et al. 1993). Finally, in another study, chlorpheniramine injected in the nucleus basalis magnocellularis or in the caudal part of the nucleus accumbens produced a significant conditioned place preference in rats (Privou et al. 1998; Zimmermann et al. 1999). Although the data obtained with H1 blockers are in accordance with the presumed inhibitory role played by histamine in reward, one must keep in mind that most of these compounds possess a high affinity for the dopamine transporter (Shishido et al. 1991; Oishi et al. 1994; Matsunaga et al. 1998). Therefore, it has been postulated that H1 antagonists potentiate the behavioral effects of drugs because they block neuronal dopamine uptake and, consequently, potentiate drug-induced rewarding effects through an increased extracellular concentration of accumbal dopamine (Suzuki et al. 1999; Lapa et al. 2005).
Manipulations of the histamine content of the brain with histaminergic drugs other than H1 blockers do not always support the hypothesis that histamine exerts inhibitory effects on reward. In agreement with that hypothesis, Suzuki et al. (1995) found that systemic administration of histidine blocked conditioned place preference induced by morphine. The same study showed that the blockade of histidine decarboxylase with α-fluoromethylhistidine, a drug that decreases the brain content of histamine, potentiates morphine place preference. However, it was found that the administration of histamine in the nucleus accumbens causes one-trial conditioned place preference in rats (Hasenohrl et al. 2001). Moreover, histidine produces place-conditioning in the gold fish (Coelho et al. 2001). Finally, the intracerebroventricular injection of histamine increases locomotor activity and dopamine release in the nucleus accumbens (Kalivas 1982; Fleckenstein et al. 1993; Galosi et al. 2001). Histamine can thus produce behavioral and neurochemical effects typically induced by addictive drugs such as psychostimulants or opioids. Indeed, all these drugs share the ability to induce conditioned place preference to stimulate locomotion and to increase dopamine levels in the nucleus accumbens (Wise and Bozarth 1987; Di Chiara et al. 2004). Taken together, these observations suggest that the disinhibition of the reward processes after the lesion of the TM does not necessarily result from a depletion of histamine in the brain.
The theory postulating that the histaminergic system from the TM exerts inhibitory effects on reward predicts that knockout mice lacking histamine would be more sensitive to the rewarding properties of cocaine (Huston et al. 1997). Therefore, the present study assessed the stimulant and rewarding effects of cocaine in knockout mice lacking histidine decarboxylase (HDC KO mice). Because histamine is synthesized by a unique enzymatic reaction with histidine decarboxylase, these mice are almost completely deficient in histamine and represent a valid model to study the role of histamine in behavior (Ohtsu et al. 2001; Parmentier et al. 2002). A first experiment characterized spontaneous locomotion and cocaine-induced hyperactivity (0, 8 and 16 mg/kg, i.p.) in wild-type and HDC KO mice. The rewarding effects of cocaine were investigated in a second experiment with the place-conditioning technique. Therefore, wild-type and HDC KO mice were injected with saline, 2, 4, 8, or 16 mg/kg cocaine (i.p.) and submitted to a biased place-conditioning protocol including four drug-pairing sessions. Locomotion recorded on the cocaine-pairing sessions was also analyzed to further characterize the locomotor-stimulant effects produced by cocaine in HDC KO mice.
Materials and methods
Animals
One hundred and eight male inbred wild-type mice and 111 histidine decarboxylase gene knockout mice (HDC KO mice) were used. The HDC KO mice were generated according to previously described procedures (Ohtsu et al. 2001). Briefly, linearized targeting construct was electroporated into R1 embryonic stem cells, derived from 129/Sv embryo (Nagy et al. 1993). The chimeric mice, generated with the confirmed embryonic stem cells, were crossed with 129/Sv mice to obtain the inbred +/− mice. The animals were kept on a 129/Sv genomic background.
At the beginning of the experiments, mice were 10 to 12 weeks old and weigh between 22 and 29 g. They were housed individually in transparent polycarbonate cages (26 × 40.5 × 20 cm) with pine sawdust bedding. Food (standard pellets, Carfil Quality BVDA, Oud-Turnhout, Belgium) and water were available ad libitum for the whole experiment. The animal room was maintained on a 12:12 h light–dark cycle (lights on at 0800 h) and an ambient temperature of 19–22°C. All experimental procedures were carried out during the light period of the light–dark cycle, between 9:00 a.m. and 1:00 p.m. In all experiments, wild-type mice served as controls for HDC KO mice and the two genotypes were simultaneously evaluated. All mice were drug-naïve, and different groups of mice were used for each dose tested. All experimental procedures were carried out in accordance with the standards of care and use of laboratory animals laid down by the European Communities Council (Directive N° 86/609/EEC, 24 November 1986). Protocols were reviewed and approved by the Animal Care Committee of the University of Liège.
Pharmacological treatment
(−)-Cocaine hydrochloride (Belgopia, Louvain-La-Neuve, Belgium) was dissolved in an isotonic saline solution (0.9% NaCl) before being administered at a volume of 0.01 ml/g body weight via the intraperitoneal route. The control treatment consisted of an equal volume of saline solution.
Spontaneous locomotion and cocaine-induced hyperactivity
Locomotor activity induced by cocaine was measured with a series of ten individual test chambers, each one comprising a square enclosure made from 0.5 cm clear acrylglas panels without base (20.5 × 20.5 × 20.5 cm). An enclosure was placed on a square plate of 0.5 cm gray acrylglas, which served as a floor, and a removable, perforated, clear acrylglas plate served as a lid. Ambulatory activity was measured by a pair of infrared light beam sensors located on each side of the enclosure at heights of 2 cm. Sensors were spaced 6.5 cm from each end of a side, so that the light beams formed a matrix of 3 × 3 squares over the surface. A mouse had to traverse the full distance (at least 6.5 cm) between the beams for each activity count. Ambulatory counts were recorded by a single personal computer to which all the testing chambers were connected. Interruptions of a single beam were not counted in the data analysis; similarly, interruptions of the intersection between perpendicularly positioned beams were not recorded. Each chamber was encased in a sound-attenuating shell (100 × 90 × 150 cm height), artificially ventilated, illuminated by a non-heating 9-W white light (350 lm), and maintained at an ambient temperature of 21–23°C. A one-way window on each shell door allowed direct visual surveillance.
Because HDC KO mice show reduced exploratory activity in an open field but normal habituation to a novel environment (Dere et al. 2004), HDC KO and control mice were habituated three times to the open field before being injected with cocaine. Therefore, for 3 days, 30 HDC KO and 30 wild-type mice received a saline injection and were immediately confined in the open field for 60 min, during which their locomotor activity was measured. After the third habituation session, mice from the two genotypes were divided in three groups (n = 10) and received an intraperitoneal injection of saline, 8 or 16 mg/kg cocaine. Immediately after the injection, mice were individually confined in the testing chamber for 60 min.
Place preference paradigm
The rewarding effects of cocaine were assessed with the place conditioning method. We used a battery of eight place preference devices purchased from Technical & Scientific Equipment, GmbH, Bad-Homburg, Germany (TSE Place Preference System, model 257000-MAU). Each chamber was illuminated by a 10-W halogen light and featured three distinct compartments with distinct visual and tactile cues: two large equally sized outer compartments (16.5 × 15 × 20 cm) separated by a smaller central compartment (6.5 × 15 × 20 cm). The central compartment served as a starting point for place preference tests. All compartments were illuminated by a dim light (2.2 W) and were made of removable opaque PVC panels, which were inserted in an aluminum frame (constituting the corners of the compartments). One of the outer compartments was colored white throughout, and the other was colored with alternate black and white 2.5-cm vertical strips, the inside of the central compartment being gray. To provide tactile cues, removable clear acrylic resin panels (homemade), whose upper surface was markedly textured, were placed on the floor of each outer compartment. The panel placed in the left compartment (striped walls) presented a relatively thinly embossed texture with 2-mm2 punches (called the “striped compartment”), whereas that inserted in the right compartment (white walls) was textured with larger 4-mm2 punches (called the “white compartment”). The central area (gray walls) was provided with a smooth floor. Entrance into and movements within the compartments were automatically recorded via an array of infrared detectors mounted every 28 mm along the entire length of the compartments walls. The infrared detectors allowed the calculation of the time spent (in seconds) in each compartment, the monitoring of the ambulatory activity that was measured in terms of the total distance traveled (in millimeters) in each compartment, and the location of the mouse in the apparatus during the session. A computer simultaneously operated the eight devices that were enclosed in sound-attenuating cubicles (60 × 40 × 40 cm).
Because a preliminary experiment showed that HDC KO mice spent more time in the striped compartment, a biased procedure (i.e., the drug was paired with the least preferred preconditioning compartment) was used to assess cocaine-induced place preference. The experiment included three phases: a preconditioning session, eight conditioning sessions, and a final test session. On the first day, 81 HDC KO mice and 78 wild-type mice were pre-exposed to the apparatus with free access to all three compartments for 20 min. Their activity was recorded and the time spent in each of the three compartments was measured. This preconditioning session served to assess unconditioned preference for one of the outer compartments (the “left-striped compartment” or the “right white compartment”) of the place conditioning apparatus. Mice that spent more than 85% of the time in one of the three compartments were eliminated from the study. The remaining mice from the two genotypes (75 HDC KO mice and 71 wild-type mice) were each divided into five groups (n = 13–15 mice/group), each group being assigned to one of the five pharmacological treatments (0, 2, 4, 8, and 16 mg/kg cocaine). During the second phase, beginning the day after the preconditioning session, animals underwent a biased conditioned place preference design including eight daily conditioning sessions. On odd days (days 1, 3, 5, and 7), mice were injected with their assigned dose of cocaine (0, 2, 4, 8, or 16 mg/kg) and confined for 20 min into the compartment that was less preferred during the preconditioning session. On even days (days 2, 4, 6, and 8), mice were injected with saline and confined for 20 min in the opposite compartment (the compartment that was preferred during the preconditioning session). When animals were placed in the drug-paired compartment (days 1, 3, 5, and 7), locomotor activity was recorded to further analyze the locomotor-activating effects of cocaine in HDC KO mice. On the third phase, which took place 24 h after the last conditioning session, mice were placed into the central area of the apparatus and given free access to all compartments for 20 min. During that period, the time spent in each compartment was automatically recorded.
Statistics
Data from the first habituation session were analyzed with a 2 × 6 mixed-model ANOVA, the genotype (HDC KO vs wild-type, two levels) being defined as a between-subject factor and the 10-min intra-session intervals (10-min blocs, six levels) as a within-subject factor. Habituation between sessions was assessed with a 2 × 3 mixed-model ANOVA, the genotype being defined as between-subject factors and the habituation sessions (three levels) as a within-subject factor. The acute locomotor effects of cocaine in the two genotypes were analyzed with a 2 × 3 × 6 mixed-model ANOVA, the genotype (HDC KO vs wild-type, two levels) and the cocaine treatment (0, 8 and 16 mg/kg cocaine, three levels) being defined as between-subject factors, and the 10-min intra-session intervals (10-min blocs, six levels) as a within-subject factor.
To analyze unconditioned preference for the compartments of the place-conditioning apparatus, time spent in the different compartments on the preconditioning session were analyzed with the Dunn–Bonferroni test. To further characterize the locomotor effects of cocaine, activity recorded on the first conditioning session was analyzed with a 2 × 5 ANOVA, the genotype (HDC KO vs wild-type, two levels) and the cocaine doses (0, 2, 4, 8, and 16 mg/kg cocaine, five levels) being defined as between-subject factors. Place preference scores were analyzed with a two-way ANOVA, the genotype (HDC KO vs wild-type, two levels) and the cocaine treatment (0, 2, 4, 8, and 16 mg/kg cocaine, five levels) being defined as between-subject factors. A score of place preference was calculated for each animal by subtracting the time spent in the drug-paired compartment during the preconditioning session from the time spent in that compartment on the test session. A score of zero would indicate an absence of change in place preference, whereas positive and negative scores would reflect preferences or aversions for the drug-paired compartment.
Where necessary, square root transformations normalized raw data before ANOVA, more nearly meeting the assumption of homogeneity of variances (after a significant Levene’s test; Zar 1999). For the sake of clarity, means of the raw values are presented in the graphs. Relevant between-mean differences were assessed via the Holm–Sidak test (Glantz 1997). Significance was always set at p < 0.05.
Results
Spontaneous locomotion and cocaine-induced hyperactivity
When exposed for the first time to the test chambers, HDC KO mice showed lower levels of locomotion than wild-type mice, especially during the beginning of the session (left panel of Fig. 1). Locomotor activity of both genotypes decreased over the habituation session, suggesting a normal intra-session habituation in histamine-deficient mice. This profile of effects is supported by a 2 × 6 mixed-model ANOVA revealing a significant effect of genotype (F 1,58 = 7.341, P < 0.01), a significant effect of the session (F 5,290 = 50.125, P < 0.001) and a significant interaction between these factors (F 5,290 = 5.458, P < 0.001). The Holm–Sidak test revealed that HDC KO mice were significantly less active than control mice during the first 10 min (P < 0.001).
To suppress differences in spontaneous activity between genotypes, wild-type and HDC KO mice were habituated daily for three times to the test chambers. Each daily habituation session lasted 60 min. The left panel shows the time course of the first habituation session in 10-min intervals. The total distance traveled during each daily session is shown on the right panel. Locomotor activity is expressed in terms of mean ± SEM number of locomotor counts. A denotes significant difference between the HDC KO mice within a 10-min block (left panel) or within a session (right panel), as yielded by Holm–Sidak tests taken at least at P < 0.05
The right panel of Fig. 1 shows habituation to the test chambers when animals were exposed daily to that environment for 3 days. The 2 × 3 mixed-model ANOVA computed on these data indicated a significant effect of genotype (F 1,58 = 4.951, P < 0.05), a significant effect of the session (F 2,116 = 38.344, P < 0.001), and a significant interaction between these factors (F 2,116 = 3.247, P < 0.05). Genotypes significantly differed from each other mainly on the first habituation session (Holm–Sidak test, P < 0.05), but not on the other sessions. The significant genotype by intersession interaction suggests that histamine-deficient mice are less active than control mice, mainly when the environment is relatively novel.
The acute locomotor effects of cocaine (saline, 8 and 16 mg/kg, i.p.) in HDC KO mice are shown in Fig. 2. A three-way ANOVA performed on these data indicated a significant effect of genotype (F 1,54 = 9.268, P < 0.01), a significant effect of the cocaine treatment (F 2,54 = 20.126, P < 0.001), a significant effect of the session (F 5,270 = 19.858, P < 0.001), a significant genotype by session interaction (F 5,270 = 2.986, P < 0.05), a significant cocaine treatment by session interaction (F 10,270 = 4.702, P < 0.001), but no cocaine by genotype interaction (F 2,54 = 2.414, P = 0.099) nor second-order interaction (F 10,270 = 4.720, P = 0.705). Post hoc tests computed over the whole session (60 min) revealed that HDC KO and wild-type mice treated with saline showed equivalent levels of locomotion (Holm–Sidak test, P = 0.977). However, locomotion of histamine-deficient mice injected with 8 mg/kg was significantly lower than that of control mice injected with the same dose (Holm–Sidak test, P < 0.05). Similarly, cocaine-induced hyperactivity was decreased in knockout mice treated with 16 mg/kg when compared with their respective wild-type mice.
Locomotor effects induced by cocaine (0, 8, and 16 mg/kg, i.p.) in wild-type and HDC KO mice. The left panel shows the time course of locomotor activity in 10-min intervals. The right panel represents mean locomotion traveled during the whole session. Locomotor activity is expressed in terms of mean ± SEM number of locomotor counts. Post hoc comparisons were performed over the whole session with the Holm–Sidak test (taken at least at P < 0.05). A denotes significant difference from the respective HDC KO group. B denotes significant difference from the respective saline-treated group within a genotype
Cocaine-induced place conditioning
During the preconditioning session, time spent in the different compartments of the place-conditioning apparatus is shown on the left panel of Fig. 3. Wild-type mice spent less time in the central area (Dunn–Bonferroni test, P < 0.01), but similar amounts of time in the white and in the striped compartments (Dunn–Bonferroni test, P > 0.10). HDC KO mice presented a different pattern of occupation of the place-conditioning apparatus. They spent more time in the striped compartment than in the white compartment (Dunn–Bonferroni test, P < 0.01). The time spent in the central compartment was similar to that spent in the white compartment (Dunn–Bonferroni test, P > 0.10). Comparisons between genotypes revealed that HDC KO mice spent significantly more time in the central compartment and less time in the white compartment than control mice (Dunn–Bonferroni test, P < 0.01). The fact that histamine-deficient mice are more anxious than wild-type mice (Dere et al. 2004) can probably explain why they prefer the central area, a compartment that is relatively smaller and darker than the two other ones. In accordance with the first experiment, the right panel of Fig. 3 shows that total activity recorded during the preconditioning session was reduced in HDC KO mice (independent t test, two-tailed, P < 0.001).
The left panel shows unconditioned preference for the different compartments of the place conditioning apparatus in wild-type and HDC KO mice. Unconditioned preference is expressed in terms of mean ± SEM time recorded in the different compartments during the preconditioning session. The right panel indicates total locomotor activity recorded in the whole apparatus during the preconditioning session in wild-type and HDC KO mice, the locomotor activity being expressed in terms of mean ± SEM distance traveled. A denotes significant difference from the HDC KO mice within a given compartment, B significant difference from the white compartment within a genotype, C significant difference from the center within a genotype, as yielded by Holm–Sidak tests taken at least at P < 0.05. D significant difference from the HDC KO mice (right panel), as indicated by an independent t test (two-tailed, P < 0.001)
The left panel of Fig. 4 shows the acute locomotor effects of cocaine recorded on the first session of the conditioning period. Two-way ANOVA indicated a significant effect of genotype (F 1,136 = 7.602, P < 0.01), a significant effect of cocaine (F 4,136 = 34.724, P < 0.01), and a significant interaction between these factors (F 4,136 = 2.456, P < 0.05). In agreement with the results from the first experiment, post hoc comparisons demonstrate that 8 and 16 mg/kg cocaine produced higher levels of activity in wild-type mice than in HDC KO mice (Holm–Sidak test, at least at P < 0.05).
Locomotor effects induced by cocaine (0, 2, 4, 8, and 16 mg/kg, i.p.) in wild-type and HDC KO mice. The left panel shows the acute locomotor effects of cocaine (0, 2, 4, 8, and 16 mg/kg, i.p.) recorded on the first cocaine-pairing session. The chronic effects of 16 mg/kg cocaine are shown in the right panel. Locomotor activity is expressed in terms of mean ± SEM distance traveled. A denotes significant difference from the respective HDC KO group, while B indicates significant difference from the first cocaine-pairing session within a genotype, as yielded by Holm–Sidak tests taken at least at P < 0.05
The right panel of Fig. 4 indicates that wild-type mice were more stimulated by 16 mg/kg cocaine than the knockout mice over the whole conditioning period (over the four cocaine-pairing sessions). However, sensitization to the locomotor effects produced by 16 mg/kg cocaine developed in both genotypes. This pattern of results is supported by a three-way mixed-model ANOVA with the genotype and the cocaine treatment (saline vs 16 mg/kg cocaine) considered as between subject-factors and the drug-pairing sessions (four levels) as a within-subject factor. This analysis revealed a significant effect of genotype (F 1,56 = 5.409, P < 0.05), a significant effect of the cocaine treatment (F 1,56 = 155.529, P < 0.0001), a significant effect of the drug-pairing sessions (F 3,168 = 9.388, P < 0.0001), a significant interaction between the genotype and the cocaine treatment (F 1,56 = 4.793, P < 0.05), a significant interaction between the drug-pairing sessions and the cocaine treatment (F 3,168 = 16.659, P < 0.0001), but no interaction between the genotype and the drug-pairing session (F 3,168 = 1.909, P = 0.129) nor second-order interaction (F 3,168 = 0.821, P < 0.483). The significant interaction between the cocaine treatment and the drug-pairing sessions indicates that both genotypes sensitized to the locomotor effects of 16 mg/kg cocaine. Post hoc comparisons demonstrate that activity on the fourth session was higher than on the first session in the cocaine-treated animals from both genotypes (Holm–Sidak test, P < 0.05). In contrast, activity on the last session was lower than on the first one in wild-type and knockout mice treated with saline, suggesting similar habituation over the sessions in both genotypes (Holm–Sidak test, P < 0.05). Wild-type mice treated with 8 mg/kg cocaine were more stimulated than knockout mice over the four drug-pairing sessions, but no sensitization developed for that dose of cocaine in neither genotypes. Similarly, there was no apparent development of sensitization after the repeated administration of 2 and 4 mg/kg cocaine (data not shown).
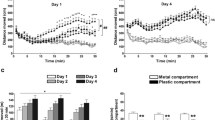
The results of the place-conditioning test are shown in Fig. 5. Cocaine produced rewarding effects in both genotypes. The two-way ANOVA calculated on the CPP scores revealed a significant effect of the cocaine treatment (F 4,136 = 5.329, P < 0.001), but no effect of genotype (F 1,136 = 0.232, P = 0.631) and no interaction between these factors (F 4,136 = 0.814, P = 0.518). Post hoc tests indicated that scores of place preference from wild-type and knockout mice treated with 8 and 16 mg/kg were significantly higher than those of the corresponding saline-treated animals (Holm–Sidak test, at least at P < 0.01). In both genotypes, the place preference scores of mice treated with 2 and 4 mg/kg were not different from their respective saline-treated mice (Holm–Sidak test, P > 0.10).
Conditioned place preference produced by cocaine (0, 2, 4, 8 and, 16 mg/kg, i.p.) in wild-type and HDC KO mice. Place preference is expressed as the mean ± SEM difference between the time spent in the drug-paired compartment before and after conditioning. A indicates significant difference from the saline-treated group within a genotype, B indicates significant difference from the group injected with 2 mg/kg cocaine within a genotype, C indicates significant difference from the group injected with 4 mg/kg cocaine within a genotype as yielded by Holm–Sidak tests taken at least at P < 0.05
Because the place conditioning scores from the wild-type animals treated with 2 and 4 mg/kg cocaine were above zero but not statistically different from the saline-treated groups, place-conditioning data were reanalyzed by comparing the time spent in the drug-paired compartment before the conditioning phase with the time spent in that compartment after the conditioning phase (Fig. 6). These data were treated with a 2 × 5 × 2 mixed-model ANOVA, the genotype and the cocaine treatment being defined as between-subject factors and the tests (preconditioning session vs place conditioning test) being defined as a within-subject factor. This type of analysis is more powerful than the 2 × 5 factorial analysis used above because it is based on within-subject comparisons that allow to detect the presence or absence of a conditioned place preference in a given group. That three-way ANOVA indicated a significant effect of the cocaine treatment (F 4,136 = 2.737, P < 0.05), a significant effect of the genotype (F 1,136 = 2.737, P < 0.05), a significant effect of the tests (F 1,136 = 36.146, P < 0.0001) and a significant interaction between the cocaine treatment and the tests (F 4,136 = 5.329, P < 0.001). There were no interactions between the cocaine treatment and the genotype (F 4,136 = 0.793, P = 0.531) nor between the tests and the genotype (F 1,136 = 0.232, P = 0.630). There was no second-order interaction (F 4,136 = 0.814, P = 0.518). The significant effect of genotype probably results from the fact that knockout mice spent, on average, less time in the drug-paired compartment than the control mice (before and after conditioning). Post hoc analyses demonstrate that HDC KO and wild-type mice treated with 8 and 16 mg/kg spent more time in the drug-paired compartment during the place-conditioning test than during the preconditioning session (Holm–Sidak test, P < 0.01). Thus, both analyses demonstrate that cocaine produced rewarding effects in both genotypes for 8 and 16 mg/kg cocaine, but not for 2 and 4 mg/kg.
Conditioned place preference produced by cocaine (0, 2, 4, 8, and 16 mg/kg, i.p.) in wild-type (left panel) and in HDC KO mice (right panel). Place preference is expressed as the mean ± SEM time spent in the drug-paired compartment after conditioning compared with the time spent in that compartment before conditioning. A denotes significant difference from the time spent in the drug-paired compartment before conditioning within a given group, as yielded by Holm–Sidak tests taken at least at P < 0.05
Discussion
In the present study, we tested the stimulant and rewarding effects of cocaine in mice lacking histamine. Because it was postulated that histamine exerts inhibitory effects on reward, histamine-deficient mice are expected to be more sensitive to the behavioral effects of cocaine than control mice. Our results show that HDC KO mice are less active than wild-type mice when placed for the first time in the test chambers. However, after three habituation sessions, they return to normal levels of activity similar to wild-type mice injected with saline. Both experiments of the present studies show that histamine knockout mice are less stimulated after the administration of 8 and 16 mg/kg cocaine, although no difference between genotypes was found after the administration of low cocaine doses, i.e., 2 and 4 mg/kg. Although less sensitive to the stimulant effects of moderate to high cocaine doses, histamine knockout mice develop a behavioral sensitization for 16 mg/kg cocaine at a similar rate to wild-type mice. Finally, the place-conditioning paradigm shows that histamine knockout and wild-type mice develop equivalent levels of place preference after conditioning with 8 and 16 mg/kg cocaine, whereas 2 and 4 mg/kg cocaine are without significant effects in both genotypes.
Histamine-deficient mice were less active than wild-type mice when exposed to a novel environment (the test chambers) for 60 min. However, they displayed a normal habituation to that environment when tested 24 h later. After three daily exposures to the test chambers, differences in activity between HDC KO and control mice had disappeared (Figs. 1 and 2). These results are in accordance with the study from Dere et al. (2004) that also reported decreased exploration in a novel environment but normal habituation in HDC KO mice. Taken together, these results confirm the role of histamine in novelty-induced arousal mechanisms (Parmentier et al. 2002). Kubota et al. (2002) also found no differences in spontaneous activity between HDC KO and wild-type mice when tested in a familiar environment (home cages) during the light period. The present results further indicate that histamine is more involved in the response to novelty than in setting basal levels of activity in a familiar environment.
According to the reward inhibitory theory, histamine is postulated to inhibit mesolimbic dopaminergic neurons by yet unknown mechanisms. Since the mesolimbic dopamine neurotransmission is involved in both the stimulant and rewarding properties of cocaine, the reward inhibitory theory entails that HDC KO mice deficient in histamine should be more sensitive to both the stimulant and rewarding effects of cocaine. The present results do not support such assumptions. On one hand, HDC KO mice were less, rather than more, sensitive to the stimulant effects of cocaine. On the other hand, relative to wild-type mice, HDC KO mice did not show a greater sensitivity to the rewarding effects of cocaine in the place-conditioning paradigm. However, the present results should be interpreted with great caution in terms of a role of histamine in the stimulant effects of cocaine. Indeed, the classical knockout approach presents important limitations, especially the possible developmental alterations that preclude such a strong conclusion. Deletion of the gene coding for HDC might have produced abnormalities in the development of the brain. These abnormalities can trigger compensatory mechanisms that are not easily detectable but can alter responsiveness to psychostimulants (Stephens et al. 2002). It is therefore difficult to know whether changes in the sensitivity to the stimulant effects of cocaine result from the absence of histamine at the time of cocaine injection or from developmental alterations in histamine and/or related neurotransmitter systems. Although HDC KO mice were fertile and showed no clear morphological or neurochemical abnormalities (Ohtsu et al. 2001), it remains possible that undetected neurochemical alterations contributed to the observed effects on cocaine sensitivity. Additional studies, if possible combining knockout and traditional pharmacological approaches, are clearly required to clarify the role of histamine in the stimulant effects of cocaine.
On the drug challenge test, HDC KO mice showed reduced levels of stimulation after the administration of 8 and 16 mg/kg cocaine, whereas the control groups of both genotypes displayed equivalent levels of activity after saline injections (Fig. 2). A similar reduction of cocaine-induced hyperactivity was observed in the place-conditioning experiment on the first cocaine-pairing session when animals received 8 and 16 mg/kg cocaine (Fig. 4). However, there was no difference between genotypes for the lower cocaine doses, i.e., 2 and 4 mg/kg. These results show that histamine-deficient mice are less stimulated than control mice by moderate (8 mg/kg) and high (16 mg/kg) doses of cocaine. The present results contrast with those obtained in studies with amphetamine and methamphetamine. Indeed, previous studies with amphetamine and methamphetamine generally reported that the blockade of the histaminergic system potentiates the stimulant effects of these psychostimulants, in agreement with the reward inhibitory theory. For example, methamphetamine-induced hyperactivity was increased in histamine-deficient mice (Kubota et al. 2002). Moreover, the inhibition of the histaminergic system with H1 antagonists (pyrilamine, chlorpheniramine) potentiated methamphetamine- and amphetamine-induced hyperactivity and stereotypies (Naylor and Costall 1971; Ito et al. 1997; Okuda et al. 2004). Conversely, the administration of agents that increase the brain histamine content was shown to diminish amphetamine- and methamphetamine-induced hyperactivity. For example, systemic injections of histidine, the precursor of histamine, reduced the locomotor stimulants effects and stereotypies produced by methamphetamine (Itoh et al. 1984, 1997). Moreover, metoprine, which blocks the activity of the enzyme that converts histamine to tele-methylhistamine and therefore increases brain histamine, was also found to decrease methamphetamine-induced hyperactivity (Itoh et al. 1984). Finally, H3 receptor inverse agonists (thioperamide, ciproxifan, and ABT-239) that enhance the release of histamine by blocking H3 autoreceptors were shown to reduce the locomotor stimulant effects of amphetamine and methamphetamine (Clapham and Kilpatrick 1994; Morisset et al. 2002; Fox et al. 2005). Together, all these studies show that histamine inhibits the locomotor stimulant effects and stereotypies induced by methamphetamine and amphetamine. However, the present results indicate that the inhibitory action of histamine cannot be generalized to other psychostimulants such as cocaine and, therefore, that histamine involves more complex mechanisms than a simple overall inhibition of reward through a modulation of mesolimbic dopamine activity. To date, only a few studies were interested in the interactions between cocaine and brain histamine, but their results agreed with those of the present study. For example, it was recently demonstrated that thioperamide, a histamine releaser, potentiated the locomotor effects of cocaine in mice (Brabant et al. 2005, 2006) and in rats (Hyytiä et al. 2003), although it is uncertain whether the increased release of histamine or other mechanisms explain the interactions between thioperamide and cocaine. Further studies are needed to investigate the role of histamine in the behavioral effects of cocaine with agents that have more specific actions on the histaminergic system than H3 inverse agonists. Indeed, H3 receptors also modulate the release of several other neurotransmitters, for example dopamine, acetylcholine, GABA, and glutamate (Brown et al. 2001), such that it is difficult to ascertain whether H3 effects are ultimately mediated by changes in histamine release.
Cocaine failed to induce higher rewarding effects in HDC KO mice relative to their wild-type littermates. On the place preference test, 8 and 16 mg/kg cocaine produced similar levels of place preference in HDC KO and wild-type mice. Additionally, lower subthreshold doses of cocaine (2 and 4 mg/kg) were without effect in both genotypes. The use of such subthreshold doses is appropriate to detect a possible greater sensitivity to cocaine reward. Indeed, cocaine-induced place preference reaches a ceiling effect abruptly. It is therefore necessary to test cocaine doses that fail to induce a place preference alone in order to demonstrate that an experimental treatment potentiates the rewarding effects of cocaine in the place-conditioning paradigm (Brabant et al. 2005; Zachariou et al. 2001). One potential limitation that could obscure the interpretation of the present place-conditioning data is that the time spent in the different compartments of the apparatus differed between genotypes during the preconditioning session. Wild-type mice spent similar amounts of time in the white and in the striped compartments. In contrast, HDC KO mice spent more time in the striped compartment than in the white and the central compartments (Fig. 3). A consequence of that differential occupation pattern of the place preference apparatus is that the time spent in the drug-paired compartment before conditioning differs between genotypes (Fig. 6). However, we do not believe that the decreased time spent in the drug-paired compartment observed in histamine-deficient mice could have masked potential rewarding effects for 2 and 4 mg/kg cocaine in these animals. Firstly, the reduced time spent in the white compartment is probably a consequence of the HDC KO mice’s preference for the central compartment rather than a result for their aversion for the white one. Indeed, Dere et al. (2004) demonstrated that histamine-deficient mice are more anxious than control mice. Therefore, HDC KO mice in the present study probably showed a preference for the central compartment that is relatively smaller and darker than the white compartment. Secondly, we reanalyzed our place-conditioning data without mice spending less than 20% of their time in the drug-paired compartment during the habituation session, i.e., after excluding the presumably more anxious mice. This analysis confirms the lack of differential sensitivity between genotypes for cocaine place preference and led to the same conclusions to that computed on all mice: cocaine produced rewarding effects in both genotypes for 8 and 16 mg/kg cocaine, but not for 2 and 4 mg/kg. For these two reasons, it is unlikely that an aversion for the drug-paired compartment has interfered with cocaine place preference observed in histamine-deficient mice.
The results of the present place-conditioning data do not support the hypothesis that histamine is involved in the inhibitory effects exerted by the tuberomammillary nucleus on reward. They are in accordance with a previous study that showed that the blockade of the histaminergic system with an H1 antagonist (chlorpheniramine) did not potentiate cocaine-induced place preference (Masukawa et al. 1993). Nevertheless, the same study found an increased place preference for methamphetamine combined with chlorpheniramine. Additionally, Suzuki et al. (1995) showed that histidine blocked the conditioned place preference induced by morphine and that the blockade of histidine decarboxylase with α-fluoromethylhistidine increased morphine-induced place preference. Therefore, it appears that the histaminergic system inhibits the rewarding effects of methamphetamine and morphine. From that point of view, the reward-inhibiting hypothesis of histamine might be restricted to the behavioral effects of some addictive drugs but might not be generalized to all forms of rewards. Indeed, several studies report data incompatible with the hypothesis that histamine exerts a simple overall inhibition on reward through a modulation of the mesolimbic dopamine system. For example, intra-accumbal histamine and systemic administration of the histamine precursor histidine were shown to be rewarding in the place-conditioning paradigm in both rats and fish (Hasenohrl et al. 2001; Coelho et al. 2001). The administration of histamine also increased locomotor activity and dopamine release in the nucleus accumbens, both effects typically induced by addictive drugs (Kalivas 1982; Fleckenstein et al. 1993; Galosi et al. 2001). It is therefore likely that the reward inhibitory effects reported after destruction of the TM are at least partially mediated by changes in neurotransmitter systems other than histamine. For example, TM lesions could produce alterations in neurochemical systems that are co-localized with the histamine system, such as GABA or glutamate neurotransmissions (Hasenohrl et al. 2001). In agreement with that hypothesis, a recent study showed that rats self-administer picrotoxin, a GABAA antagonist, into the supramammillary nucleus (Ikemoto 2005), a hypothalamic area belonging to the diffuse part of the tuberomammillary nucleus (Ericson et al. 1987). These latter results suggest that the blockade of GABAA receptors in the supramammillary nucleus is reinforcing and that the brain reward circuitry is tonically inhibited through the activation of supramammillary GABAA receptors (Ikemoto 2005). Other studies investigating the rewarding effects of agents directly infused in the TM need to be conducted to determine the neurochemical mechanisms involved in the reward inhibitory function of the TM.
In summary, our data confirm previous results demonstrating that histidine decarboxylase knockout mice show reduced exploratory behaviors but normal habituation to a novel environment. Furthermore, the locomotor effects of cocaine were slightly attenuated in these mice, but they showed no differences in cocaine-induced conditioned place preference. In disagreement with the postulated reward inhibitory function of histamine, histamine-deficient mice did not show a greater sensitivity to the locomotor stimulant and rewarding effects of cocaine.
References
Arrang JM, Garbarg M, Schwartz JC (1983) Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 302:832–837
Beardsley PM, Balster RL (1992) The intravenous self-administration of antihistamines by rhesus monkeys. Drug Alcohol Depend 30:117–126
Bergman J, Spealman RD (1986) Some behavioral effects of histamine H1 antagonists in squirrel monkeys. J Pharmacol Exp Ther 239:104–110
Brabant C, Charlier Y, Quertemont E, Tirelli E (2005) The H3 antagonist thioperamide reveals conditioned preference for a context associated with an inactive small dose of cocaine in C57BL/6J mice. Behav Brain Res 160:161–168
Brabant C, Quertemont E, Tirelli E (2006) Effects of the H3-receptor inverse agonist thioperamide on the psychomotor effects induced by acutely and repeatedly given cocaine in C57BL/6J mice. Pharmacol Biochem Behav 83:561–569
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672
Clapham J, Kilpatrick GJ (1994) Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol 259:107–114
Coelho JL, Medalha CC, Mattioli R (2001) Analysis of the effects of CPA and L-histidine on goldfish tested on a conditioned place preference model. Behav Brain Res 124:161–165
Cohn CK, Ball GG, Hirsch J (1973) Histamine: effect on self-stimulation. Science 180:757–758
Dere E, Souza-Silva MA, Topic B, Spieler RE, Haas HL, Huston JP (2003) Histidine-decarboxylase knockout mice show deficient nonreinforced episodic object memory, improved negatively reinforced water–maze performance, and increased neo- and ventro-striatal dopamine turnover. Learn Mem 10:510–519
Dere E, Souza-Silva MA, Spieler RE, Lin JS, Ohtsu H, Haas HL, Huston JP (2004) Changes in motoric, exploratory and emotional behaviours and neuronal acetylcholine content and 5-HT turnover in histidine decarboxylase-KO mice. Eur J Neurosci 20:1051–1058
Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47(Suppl 1):227–241
Ericson H, Watanabe T, Kohler C (1987) Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol 263:1–24
Fleckenstein AE, Lookingland KJ, Moore KE (1993) Activation of mesolimbic dopaminergic neurons following central administration of histamine is mediated by H1 receptors. Naunyn Schmiedebergs Arch Pharmacol 347:50–54
Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE et al (2005) Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 313:176–190
Galosi R, Lenard L, Knoche A, Haas H, Huston JP, Schwarting RK (2001) Dopaminergic effects of histamine administration in the nucleus accumbens and the impact of H1-receptor blockade. Neuropharmacology 40:624–633
Glantz S (1997) A primer of biostatistics, 4th edn. McGraw-Hill, New York
Haas H, Panula P (2003) The role of histamine and the tuberomammillary nucleus in the nervous system. Nat Rev Neurosci 4:121–130
Hasenohrl RU, Kuhlen A, Frisch C, Galosi R, Brandao ML, Huston JP (2001) Comparison of intra-accumbens injection of histamine with histamine H1-receptor antagonist chlorpheniramine in effects on reinforcement and memory parameters. Behav Brain Res 124:203–211
Huston JP, Wagner U, Hasenohrl RU (1997) The tuberomammillary nucleus projections in the control of learning, memory and reinforcement processes: evidence for an inhibitory role. Behav Brain Res 83:97–105
Hyytiä P, Bäckström P, Piepponen P, Ahtee L (2003) Histamine H3-receptor antagonist thioperamide potentiates behavioral effets of cocaine. Eur J Pharm Sci 19:S21–S24
Ikemoto S (2005) The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology 30:1088–1095
Ito C, Onodera K, Watanabe T, Sato M (1997) Effects of histamine agents on methamphetamine-induced stereotyped behavior and behavioral sensitization in rats. Psychopharmacology (Berl) 130:362–367
Itoh Y, Nishibori M, Oishi R, Saeki K (1984) Neuronal histamine inhibits methamphetamine-induced locomotor hyperactivity in mice. Neurosci Lett 48:305–309
Kalivas PW (1982) Histamine-induced arousal in the conscious and pentobarbital-pretreated rat. J Pharmacol Exp Ther 222:37–42
Kubota Y, Ito C, Sakurai E, Sakurai E, Watanabe T, Ohtsu H (2002) Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J Neurochem 83:837–845
Lapa GB, Mathews TA, Harp J, Budygin EA, Jones SR (2005) Diphenylpyraline, a histamine H1 receptor antagonist, has psychostimulant properties. Eur J Pharmacol 506:237–240
Leurs R, Bakker RA, Timmerman H, de Esch IJ (2005) The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 4:107–120
Masukawa Y, Suzuki T, Misawa M (1993) Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology (Berl) 111:139–143
Matsunaga K, Sato T, Shuto H, Tsuruta Y, Suemaru K, Gomita Y, Oishi R (1998) Inhibition of neuronal dopamine uptake by some antiallergic drugs. Eur J Pharmacol 350:165–169
Morisset S, Pilon C, Tardivel-Lacombe J, Weinstein D, Rostene W, Betancur C, Sokoloff P, Schwartz JC, Arrang JM (2002) Acute and chronic effects of methamphetamine on tele-methylhistamine levels in mouse brain: selective involvement of the D(2) and not D(3) receptor. J Pharmacol Exp Ther 300:621–628
Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90:8424–8428
Naylor RJ, Costall B (1971) The relationship between the inhibition of dopamine uptake and the enhancement of amphetamine stereotypy. Life Sci I 10:909–915
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E et al (2001) Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett 502:53–56
Oishi R, Shishido S, Yamori M, Saeki K (1994) Comparison of the effects of eleven histamine H1-receptor antagonists on monoamine turnover in the mouse brain. Naunyn Schmiedebergs Arch Pharmacol 349:140–144
Okuda T, Ito Y, Nakagawa N, Hishinuma T, Tsukamoto H, Iwabuchi K, Watanabe T, Kitaichi K, Goto J, Yanai K (2004) Drug interaction between methamphetamine and antihistamines: behavioral changes and tissue concentrations of methamphetamine in rats. Eur J Pharmacol 505:135–144
Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS (2002) Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep–wake control. J Neurosci 22:7695–7711
Privou C, Knoche A, Hasenohrl RU, Huston JP (1998) The H1- and H2-histamine blockers chlorpheniramine and ranitidine applied to the nucleus basalis magnocellularis region modulate anxiety and reinforcement related processes. Neuropharmacology 37:1019–1032
Sannerud CA, Kaminski BJ, Griffiths RR (1995) Maintenance of H1 antagonists self-injection in baboons. Exp Clin Psychopharmacol 3:26–32
Shishido S, Oishi R, Saeki K (1991) In vivo effects of some histamine H1-receptor antagonists on monoamine metabolism in the mouse brain. Naunyn Schmiedebergs Arch Pharmacol 343:185–189
Stephens DN, Mead AN, Ripley TL (2002) Studying the neurobiology of stimulant and alcohol abuse and dependence in genetically manipulated mice. Behav Pharmacol 13:327–345
Suzuki T, Masukawa Y, Misawa M (1990) Drug interactions in the reinforcing effects of over-the-counter cough syrups. Psychopharmacology (Berl) 102:438–442
Suzuki T, Masukawa Y, Shiozaki Y, Misawa M (1991) Potentiation of pentazocine conditioned place preference by tripelennamine in rats. Psychopharmacology (Berl) 105:9–12
Suzuki T, Takamori K, Misawa M, Onodera K (1995) Effects of the histaminergic system on the morphine-induced conditioned place preference in mice. Brain Res 675:195–202
Suzuki T, Mori T, Tsuji M, Nomura M, Misawa M, Onodera K (1999) Evaluation of the histamine H1-antagonist-induced place preference in rats. Jpn J Pharmacol 81:332–338
Wagner U, Segura-Torres P, Weiler T, Huston JP (1993a) The tuberomammillary nucleus region as a reinforcement inhibiting substrate: facilitation of ipsihypothalamic self-stimulation by unilateral ibotenic acid lesions. Brain Res 613:269–274
Wagner U, Weiler HT, Huston JP (1993b) Amplification of rewarding hypothalamic stimulation following a unilateral lesion in the region of the tuberomammillary nucleus. Neuroscience 52:927–932
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR (2001) Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology 24:576–589
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Zimmermann P, Privou C, Huston JP (1999) Differential sensitivity of the caudal and rostral nucleus accumbens to the rewarding effects of a H1-histaminergic receptor blocker as measured with place-preference and self-stimulation behavior. Neuroscience 94:93–103
Acknowledgements
The present research was supported by the Belgian National Funds for Scientific Research (FNRS), grant F.R.F.C. N°2.4533.02F to Ezio Tirelli.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brabant, C., Quertemont, E., Anaclet, C. et al. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology 190, 251–263 (2007). https://doi.org/10.1007/s00213-006-0603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0603-0