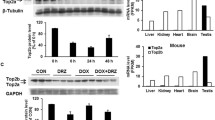
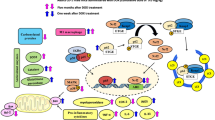
Abstract
Purpose
The iron chelator Dp44mT is a potent topoisomerase IIα inhibitor with novel anticancer activity. Doxorubicin (Dox), the current front-line therapy for breast cancer, induces a dose-limiting cardiotoxicity, in part through an iron-mediated pathway. We tested the hypothesis that Dp44mT can improve clinical outcomes of treatment with Dox by alleviating cardiotoxicity.
Methods
The general cardiac and renal toxicities induced by Dox were investigated in the presence and absence of Dp44mT. The iron chelating cardioprotectant Dexrazoxane (Drz), which is approved for this indication, was used as a positive control. In vitro studies were carried out with H9c2 rat cardiomyocytes and in vivo studies were performed using spontaneously hypertensive rats.
Results
Testing of the GI50 profile of Dp44mT in the NCI-60 panel confirmed activity against breast cancer cells. An acute, toxic dose of Dox caused the predicted cellular and cardiac toxicities, such as cell death and DNA damage in vitro and elevated cardiac troponin T levels, tissue damage, and apoptosis in vivo. Dp44mT alone caused insignificant changes in hematological and biochemical indices in rats, indicating that Dp44mT is not significantly cardiotoxic as a single agent. In contrast to Drz, Dp44mT failed to mitigate Dox-induced cardiotoxicity in vivo.
Conclusions
We conclude that although Dp44mT is a potent iron chelator, it is unlikely to be an appropriate cardioprotectant against Dox-induced toxicity. However, it should continue to be evaluated as a potential anticancer agent as it has a novel mechanism for inhibiting the growth of a broad range of malignant cell types while exhibiting very low intrinsic toxicity to healthy tissues.




Similar content being viewed by others
References
Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE (2007) Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother 8:1039–1058
Bolli R (2001) Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33:1897–1918
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y (2008) gammaH2AX and cancer. Nat Rev Cancer 8:957–967
Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM (2009) Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis 30:1686–1695
Hasinoff BB, Patel D (2009) The iron chelator Dp44mT does not protect myocytes against doxorubicin. J Inorg Biochem 103:1093–1101
Herman EH, Zhang J, Chadwick DP, Ferrans VJ (2000) Comparison of the protective effects of amifostine and dexrazoxane against the toxicity of doxorubicin in spontaneously hypertensive rats. Cancer Chemother Pharmacol 45:329–334
Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G (1991) Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69:1476–1486
Hoke EM, Maylock CA, Shacter E (2005) Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med 39:403–411
Imondi AR (1998) Preclinical models of cardiac protection and testing for effects of dexrazoxane on doxorubicin antitumor effects. Semin Oncol 25:22–30
Kaiserova H, den Hartog GJ, Simunek T, Schroterova L, Kvasnickova E, Bast A (2006) Iron is not involved in oxidative stress-mediated cytotoxicity of doxorubicin and bleomycin. Br J Pharmacol 149:920–930
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234–235:119–124
Khan RS, Drosatos K, Goldberg IJ (2010) Creating and curing fatty hearts. Curr Opin Clin Nutr Metab Care 13:145–149
Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF (2007) Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 67:8839–8846
McClendon AK, Osheroff N (2007) DNA topoisomerase II, genotoxicity, and cancer. Mutat Res 623:83–97
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
Mitra MS, Donthamsetty S, White B, Mehendale HM (2008) High fat diet-fed obese rats are highly sensitive to doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol 231:413–422
Myers C (1998) The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol 25:10–14
Qiu Y, Rizvi A, Tang XL, Manchikalapudi S, Takano H, Jadoon AK, Wu WJ, Bolli R (1997) Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol 273:H2931–H2936
Rao VA, Fan AM, Meng L, Doe CF, North PS, Hickson ID, Pommier Y (2005) Phosphorylation of BLM, dissociation from topoisomerase IIIalpha, and colocalization with gamma-H2AX after topoisomerase I-induced replication damage. Mol Cell Biol 25:8925–8937
Rao VA, Klein SR, Agama KK, Toyoda E, Adachi N, Pommier Y, Shacter EB (2009) The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res 69:948–957
Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Rahmanto YS, Sheftel AD, Ponka P (2010) Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci USA 107:10775–10782
Rogakou EP, Boon C, Redon C, Bonner WM (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146:905–916
Sardao VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB (2009) Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemother Pharmacol 64:811–827
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Tatlidede E, Sehirli O, Velioglu-Ogunc A, Cetinel S, Yegen BC, Yarat A, Suleymanoglu S, Sener G (2009) Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res 43:195–205
Wallace KB, Hausner E, Herman E, Holt GD, MacGregor JT, Metz AL, Murphy E, Rosenblum IY, Sistare FD, York MJ (2004) Serum troponins as biomarkers of drug-induced cardiac toxicity. Toxicol Pathol 32:106–121
Whitnall M, Howard J, Ponka P, Richardson DR (2006) A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA 103:14901–14906
Wonders KY, Hydock DS, Greufe S, Schneider CM, Hayward R (2009) Endurance exercise training preserves cardiac function in rats receiving doxorubicin and the HER-2 inhibitor GW2974. Cancer Chemother Pharmacol 64:1105–1113
Yan T, Deng S, Metzger A, Godtel-Armbrust U, Porter AC, Wojnowski L (2009) Topoisomerase II{alpha}-dependent and -independent apoptotic effects of dexrazoxane and doxorubicin. Mol Cancer Ther 8(5):1075–1085
Yuan J, Lovejoy DB, Richardson DR (2004) Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood 104:1450–1458
Acknowledgments
This study was funded by the intramural research program of the Food and Drug Administration and the National Cancer Institute. We thank Dr. Yves Pommier (NCI) for helpful discussions and Dr. Melanie Simpson (NCI) for critical reading of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Food and Drug Administration.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2011_1587_MOESM1_ESM.eps
Fig. S1 Anticancer cytotoxicity profile of Dp44mT. The NCI 60 panel of cancer cell lines was treated with increasing concentrations of Dp44mT in order to evaluate the relative sensitivities to the drug in different tissue types. Cell lines are depicted graphically by their tissue of origin. Red asterisks indicate tissue types that were most sensitive to the drug. Supplementary material 1 (EPS 11951 kb)
280_2011_1587_MOESM2_ESM.eps
Fig. S2 Anticancer cytotoxic profile of Dp44mT. The GI50 value for each cell line and tissue type tested is shown. Bars protruding to the right indicate increased sensitivity to Dp44mT while bars protruding to the left indicate increased resistance. (EPS 4519 kb)
Rights and permissions
About this article
Cite this article
Rao, V.A., Zhang, J., Klein, S.R. et al. The iron chelator Dp44mT inhibits the proliferation of cancer cells but fails to protect from doxorubicin-induced cardiotoxicity in spontaneously hypertensive rats. Cancer Chemother Pharmacol 68, 1125–1134 (2011). https://doi.org/10.1007/s00280-011-1587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1587-y