Abstract
Objectives
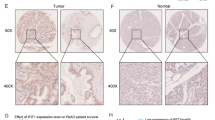
Lysyl oxidase-like 2 (LOXL2) plays a part in epithelial–mesenchymal transition (EMT) by stabilizing the transcription factor SNAI1. Previous studies showed that LOXL2 is one of the most highly and specifically upregulated genes in pancreatic cancer. LOXL2 was also found to be strongly upregulated in the secretome of established pancreatic cancer cell lines. To get more insight into the aggressive growth and infiltrating nature of pancreatic cancer, we evaluated the functional role of LOXL2 in pancreatic cancer cells.
Methods
Gene inhibition by small interfering RNAs was used to silence LOXL2 in pancreatic cancer cell lines MiaPaCa-2 and Panc1. Cell death, proliferation, and morphology of transfected cells were determined. Cell characteristics under cell stress and gemcitabine treatment were analyzed. Gene expression analysis of transfected cells by DNA microarray was used to understand the processes of chemosensitization.
Results
Silencing of LOXL2 in pancreatic cancer cells resulted in an augmented sensitivity toward gemcitabine treatment, with significantly elevated cell death and reduced viable cells. However, transfection had no direct effect on morphology or growth pattern of Mia PaCa-2 and Panc1 cell lines. Gene expression analysis identified among others the transcription factor E2F5 as possible target of LOXL2.
Conclusions
Gene inhibition of the EMT regulatory gene LOXL2 resulted in a distinct sensitization toward gemcitabine. Additionally, gene expression analysis showed a role for LOXL2 in the regulation of different transcription factors associated with invasion and metastasis. Our results suggest that the improved response toward chemotherapy in LOLX2-silenced pancreatic cancer cells is possibly mediated by the transcription factor E2F5.




Similar content being viewed by others
References
Lowenfels AB, Maisonneuve P (2006) Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 20:197–209
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics. 2008. CA Cancer J Clin 58:71–96
Eccles SA, Welch DR (2007) Metastasis: recent discoveries and novel treatment strategies. Lancet 369:1742–1757
Wolff RA, Chiao P, Lenzi R, Pisters PW, Lee JE, Janjan NA, Crane CH, Evans DB, Abbruzzese JL (2000) Current approaches and future strategies for pancreatic carcinoma. Invest New Drugs 18:43–56
Maki JM, Tikkanen H, Kivirikko KI (2001) Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol 20:493–496
Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K (2003) Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta 1647:220–224
Smith-Mungo LI, Kagan HM (1998) Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16:387–398
Payne SL, Hendrix MJ, Kirschmann DA (2007) Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem 101:1338–1354
Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G (2003) Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res 63:1657–1666
Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ (2002) A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res 62:4478–4483
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG (2007) Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res 13:4769–4776
Ban S, Ishikawa K, Kawai S, Koyama-Saegusa K, Ishikawa A, Shimada Y, Inazawa J, Imai T (2005) Potential in a single cancer cell to produce heterogeneous morphology, radiosensitivity and gene expression. J Radiat Res (Tokyo) 46:43–50
Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, Parker MI, Csiszar K (2007) Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer 46:644–655
Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A (2008) Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res 68:4541–4550
Grutzmann R, Foerder M, Alldinger I, Staub E, Brummendorf T, Ropcke S, Li X, Kristiansen G, Jesnowski R, Sipos B, Lohr M, Luttges J, Ockert D, Kloppel G, Saeger HD, Pilarsky C (2003) Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch 443:508–517
Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, Pandey A (2006) Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics 5:157–171
Pilarsky C, Ammerpohl O, Sipos B, Dahl E, Hartmann A, Wellmann A, Braunschweig T, Lohr M, Jesnowski R, Friess H, Wente MN, Kristiansen G, Jahnke B, Denz A, Ruckert F, Schackert HK, Kloppel G, Kalthoff H, Saeger HD, Grutzmann R (2008) Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. J Cell Mol Med 12:2823–2835
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
Peinado H, Portillo F, Cano A (2005) Switching on-off snail: LOXL2 versus GSK3beta. Cell Cycle 4:1749–1752
Peinado H, Iglesias-de DC, la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J 24:3446–3458
Jesnowski R, Furst D, Ringel J, Chen Y, Schrodel A, Kleeff J, Kolb A, Schareck WD, Lohr M (2005) Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest 85:1276–1291
Grutzmann R, Pilarsky C, Ammerpohl O, Luttges J, Bohme A, Sipos B, Foerder M, Alldinger I, Jahnke B, Schackert HK, Kalthoff H, Kremer B, Kloppel G, Saeger HD (2004) Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia 6:611–622
Madden ME, Sarras MP Jr (1988) Morphological and biochemical characterization of a human pancreatic ductal cell line (PANC-1). Pancreas 3:512–528
Madden ME, Heaton KM, Huff JK, Sarras MP Jr (1989) Comparative analysis of a human pancreatic undifferentiated cell line (MIA PaCa-2) to acinar and ductal cells. Pancreas 4:529–537
Lo M, Ling V, Wang YZ, Gout PW (2008) The xc-cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer 99:464–472
Wang C, Xiao Y, Hu Z, Chen Y, Liu N, Hu G (2008) PEG10 directly regulated by E2Fs might have a role in the development of hepatocellular carcinoma. FEBS Lett 582:2793–2798
Okabe H, Satoh S, Furukawa Y, Kato T, Hasegawa S, Nakajima Y, Yamaoka Y, Nakamura Y (2003) Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res 63:3043–3048
Stevens TH, Forgac M (1997) Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol 13:779–808
Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Sennoune SR, Luo D, Martinez-Zaguilan R (2004) Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys 40:185–206
King LM, Francomano CA (2001) Characterization of a human gene encoding nucleosomal binding protein NSBP1. Genomics 71:163–173
Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA (2007) Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer 43:415–432
Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H, Schultz PG, Powell SM, Moskaluk CA, Frierson HF Jr, Hampton GM (2001) Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 61:7388–7393
Spychala J, Lazarowski E, Ostapkowicz A, Ayscue LH, Jin A, Mitchell BS (2004) Role of estrogen receptor in the regulation of ecto-5'-nucleotidase and adenosine in breast cancer. Clin Cancer Res 10:708–717
Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou Z, Zhou P (2008) Ecto-5'-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol 134:365–372
Mujoomdar M, Bennett A, Hoskin D, Blay J (2004) Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol 201:429–438
McMahon B, Kwaan HC (2008) The plasminogen activator system and cancer. Pathophysiol Haemost Thromb 36:184–194
Malo M, Charriere-Bertrand C, Chettaoui C, Fabre-Guillevin E, Maquerlot F, Lackmy A, Vallee B, Delaplace F, Barlovatz-Meimon G (2006) The PAI-1 swing: microenvironment and cancer cell migration. C R Biol 329:919–927
Czekay RP, Loskutoff DJ (2004) Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med (Maywood) 229:1090–1096
Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G (2005) Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem 280:18211–18220
Le Cam L, Polanowska J, Fabbrizio E, Olivier M, Philips A, Ng Eaton E, Classon M, Geng Y, Sardet C (1999) Timing of cyclin E gene expression depends on the regulated association of a bipartite repressor element with a novel E2F complex. Embo J 18:1878–1890
Chen CR, Kang Y, Siegel PM, Massague J (2002) E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 110:19–32
Valladares A, Hernandez NG, Gomez FS, Curiel-Quezada E, Madrigal-Bujaidar E, Vergara MD, Martinez MS, Arenas Aranda DJ (2006) Genetic expression profiles and chromosomal alterations in sporadic breast cancer in Mexican women. Cancer Genet Cytogenet 170:147–151
Wang A, Li CJ, Reddy PV, Pardee AB (2005) Cancer chemotherapy by deoxynucleotide depletion and E2F-1 elevation. Cancer Res 65:7809–7814
Author information
Authors and Affiliations
Corresponding author
Additional information
Robert Grützmann and Christian Pilarsky share the senior authorship for this work.
Felix Rükert and Peer Joensson contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Rückert, F., Joensson, P., Saeger, HD. et al. Functional analysis of LOXL2 in pancreatic carcinoma. Int J Colorectal Dis 25, 303–311 (2010). https://doi.org/10.1007/s00384-009-0853-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0853-5