Abstract
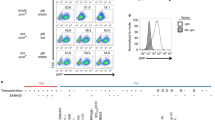
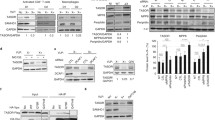
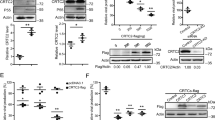
TRIM5α has been identified as the main restriction factor responsible for resistance of Old World monkey cells to HIV-1 infection. The precise mechanism of viral inhibition by TRIM5α remains elusive but appears to occur in multiple ways. Here, we report that rhesus monkey TRIM5α (TRIM5αrh) can represses HIV-1 LTR promoter activity by negatively regulating TAK1/TAB1/TAB2/TAB3-complex-mediated NF-κB activation when TRIM5αrh is overexpressed. We show that the overexpressed TRIM5αrh can interact with the TAK1/TAB1/TAB2/TAB3 complex by binding to TAB1 and promotes the degradation of TAB2 within the complex via the lysosomal degradation pathway. Subsequently, TRIM5αrh lowers the IKKα protein level and inhibits NF-κB p65 phosphorylation, and knockdown of TRIM5αrh expression by small interfering RNA in TRIM5αrh-overexpressing cells can abolish this inhibition. Finally, the inhibition of p65 phosphorylation results in the repression of HIV-1 LTR promoter activity. Taken together, these findings indicate that TRIM5αrh plays a previously unrecognized role in repressing HIV-1 transcription by inhibiting TAK1/TAB1/TAB2/TAB3-complex-mediated NF-κB activation when TRIM5αrh is overexpressed.






Similar content being viewed by others
References
Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA (1986) Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291
Akira S (2006) TLR signaling. Curr Topics microbiol immunol 311:1–16
Anderson JL, Campbell EM, Wu X, Vandegraaff N, Engelman A, Hope TJ (2006) Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol 80:9754–9760
Berube J, Bouchard A, Berthoux L (2007) Both TRIM5alpha and TRIMCyp have only weak antiviral activity in canine D17 cells. Retrovirology 4:68
Campbell KJ, Perkins ND (2004) Post-translational modification of RelA(p65) NF-kappaB. Biochem Soc Transact 32:1087–1089
Chatterji U, Bobardt MD, Gaskill P, Sheeter D, Fox H, Gallay PA (2006) Trim5alpha accelerates degradation of cytosolic capsid associated with productive HIV-1 entry. J Biol Chem 281:37025–37033
Chen G, Goeddel DV (2002) TNF-R1 signaling: a beautiful pathway. Science (New York, NY 296:1634–1635
Cheung PC, Nebreda AR, Cohen P (2004) TAB3, a new binding partner of the protein kinase TAK1. Biochem J 378:27–34
Connor RI, Chen BK, Choe S, Landau NR (1995) Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351–361
Faustman D, Davis M (2010) TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev 9:482–493
Heller RA, Kronke M (1994) Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol 126:5–9
Hiscott J, Kwon H, Genin P (2001) Hostile takeovers: viral appropriation of the NF-kappaB pathway. J Clin Investig 107:143–151
Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J (1996) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 271:28745–28748
Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T (2003) The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J Biol Chem 278:919–926
Lawrence T, Bebien M, Liu GY, Nizet V, Karin M (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434:1138–1143
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev 2:725–734
Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151
Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML (2004) Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol 172:6336–6344
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K (1999) The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252–256
Pereira LA, Bentley K, Peeters A, Churchill MJ, Deacon NJ (2000) A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Res 28:663–668
Perez-Caballero D, Hatziioannou T, Yang A, Cowan S, Bieniasz PD (2005) Human tripartite motif 5alpha domains responsible for retrovirus restriction activity and specificity. J Virol 79:8969–8978
Perron MJ, Stremlau M, Song B, Ulm W, Mulligan RC, Sodroski J (2004) TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci U S A 101:11827–11832
Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, Bisiaux A, Albert ML, Strambio-De-Castillia C, Mothes W, Pizzato M, Grutter MG, Luban J (2011) TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature 472:361–365
Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A (2001) The tripartite motif family identifies cell compartments. EMBO J 20:2140–2151
Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J (1995) Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors. Microbiol Rev 59:481–505
Sakuma R, Noser JA, Ohmine S, Ikeda Y (2007) Rhesus monkey TRIM5alpha restricts HIV-1 production through rapid degradation of viral Gag polyproteins. Nat Med 13:631–635
Sakuma R, Ohmine S, Ikeda Y (2010) Determinants for the rhesus monkey TRIM5alpha-mediated block of the late phase of HIV-1 replication. J Biol Chem 285:3784–3793
Sakurai H, Miyoshi H, Mizukami J, Sugita T (2000) Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett 474:141–145
Sakurai H, Nishi A, Sato N, Mizukami J, Miyoshi H, Sugita T (2002) TAK1-TAB1 fusion protein: a novel constitutively active mitogen-activated protein kinase kinase kinase that stimulates AP-1 and NF-kappaB signaling pathways. Biochem Biophys Res Commun 297:1277–1281
Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I (2003) Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem 278:36916–36923
Shi M, Deng W, Bi E, Mao K, Ji Y, Lin G, Wu X, Tao Z, Li Z, Cai X, Sun S, Xiang C, Sun B (2008) TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol 9:369–377
Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, Nishida E, Matsumoto K (1996) TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science (New York, NY) 272:1179–1182
Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J (2004) The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853
Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J (2006) Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA 103:5514–5519
Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol cell 5:649–658
Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB (2003) TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol 326:105–115
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14
Tareen Semih U, Emerman M (2011) Human Trim5α has additional activities that are uncoupled from retroviral capsid recognition. Virology 409:119–120
Wang CY, Cusack JC Jr, Liu R, Baldwin AS Jr (1999) Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 5:412–417
Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ (2006) Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci USA 103:7465–7470
Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K (1995) Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science (New York, NY) 270:2008–2011
Yamauchi K, Wada K, Tanji K, Tanaka M, Kamitani T (2008) Ubiquitination of E3 ubiquitin ligase TRIM5 alpha and its potential role. FEBS J 275:1540–1555
Yoshimi R, Chang TH, Wang H, Atsumi T, Morse HC 3rd, Ozato K (2009) Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J Immunol 182:7527–7538
Zha J, Han KJ, Xu LG, He W, Zhou Q, Chen D, Zhai Z, Shu HB (2006) The Ret finger protein inhibits signaling mediated by the noncanonical and canonical IkappaB kinase family members. J Immunol 176:1072–1080
Acknowledgments
We thank Dr. Hong-Bing Shu (Wuhan University, Wuhan, China) for providing the Flag-TAB2 and Flag-TAB3 plasmids, Dr. Zheng-Li Shi (Wuhan Institute of Virology, CAS, Wuhan, China) for providing pVpack-GP and pVpack-VSV-G plasmids, Dr. Qun-Yuan Xu (Capital Medical University, Beijing, China) for providing the pLPCX plasmid, Prof. Tian-Xian Li (Wuhan Institute of Virology, CAS, Wuhan, China) for providing the marmoset B-lymphoblastoid (B95-8) and fetal rhesus monkey kidney (FRhk-4) cell lines. This work was supported by a grant of the Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period of China (2008ZX10001-002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, J., Shen, XH., Qiu, H. et al. Rhesus monkey TRIM5α represses HIV-1 LTR promoter activity by negatively regulating TAK1/TAB1/TAB2/TAB3-complex-mediated NF-κB activation. Arch Virol 156, 1997–2006 (2011). https://doi.org/10.1007/s00705-011-1097-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-1097-6