Abstract
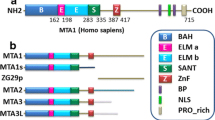
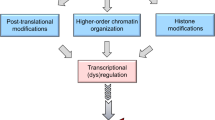
Although the functional significance of the metastasic tumor antigen (MTA) family of chromatin remodeling proteins in the pathobiology of cancer is fairly well recognized, the physiological role of MTA proteins continues to be an understudied research area and is just beginning to be recognized. Similar to cancer cells, MTA1 also modulates the expression of target genes in normal cells either by acting as a corepressor or coactivator. In addition, physiological functions of MTA proteins are likely to be influenced by its differential expression, subcellular localization, and regulation by upstream modulators and extracellular signals. This review summarizes our current understanding of the physiological functions of the MTA proteins in model systems. In particular, we highlight recent advances of the role MTA proteins play in the brain, eye, circadian rhythm, mammary gland biology, spermatogenesis, liver, immunomodulation and inflammation, cellular radio-sensitivity, and hematopoiesis and differentiation. Based on the growth of knowledge regarding the exciting new facets of the MTA family of proteins in biology and medicine, we speculate that the next burst of findings in this field may reveal further molecular regulatory insights of non-redundant functions of MTA coregulators in the normal physiology as well as in pathological conditions outside cancer.


Similar content being viewed by others
References
Toh, Y., Pencil, S. D., & Nicolson, G. L. (1994). A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. Journal of Biological Chemistry, 269(37), 22958–22963.
Toh, Y., Pencil, S. D., & Nicolson, G. L. (1995). Analysis of the complete sequence of the novel metastasis-associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene, 159(1), 97–104.
Mazumdar, A., Wang, R. A., Mishra, S. K., Adam, L., Bagheri-Yarmand, R., Mandal, M., et al. (2001). Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biology, 3(1), 30–37.
Li, W., Ma, L., Zhao, J., Liu, X., Li, Z., & Zhang, Y. (2009). Expression profile of MTA1 in adult mouse tissues. Tissue and Cell, 41(6), 390–399.
Liu, J., Xu, D., Wang, H., Zhang, Y., Chang, Y., Zhang, J., et al. (2014). The subcellular distribution and function of MTA1 in cancer differentiation. Oncotarget, 5(13), 5153–5164.
Li, D. Q., Pakala, S. B., Nair, S. S., Eswaran, J., & Kumar, R. (2012). Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Research, 72(2), 387–394.
Xue, Y., Wong, J., Moreno, G. T., Young, M. K., Cote, J., & Wang, W. (1998). NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Molecular Cell, 2(6), 851–861.
Zhang, Y., Ng, H. H., Erdjument-Bromage, H., Tempst, P., Bird, A., & Reinberg, D. (1999). Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes & Development, 13(15), 1924–1935.
Yao, Y. L., & Yang, W. M. (2003). The metastasis-associated proteins 1 and 2 form distinct protein complexes with histone deacetylase activity. Journal of Biological Chemistry, 278(43), 42560–42568.
Bowen, N. J., Fujita, N., Kajita, M., & Wade, P. A. (2004). Mi-2/NuRD: multiple complexes for many purposes. Biochimica et Biophysica Acta, 1677(1–3), 52–57.
Denslow, S. A., & Wade, P. A. (2007). The human Mi-2/NuRD complex and gene regulation. Oncogene, 26(37), 5433–5438.
Millard, C. J., Watson, P. J., Celardo, I., Gordiyenko, Y., Cowley, S. M., Robinson, C. V., et al. (2013). Class I HDACs share a common mechanism of regulation by inositol phosphates. Molecular Cell, 51(1), 57–67.
Herman, M. A., Ch’ng, Q., Hettenbach, S. M., Ratliff, T. M., Kenyon, C., & Herman, R. K. (1999). EGL-27 is similar to a metastasis-associated factor and controls cell polarity and cell migration in C. elegans. Development, 126(5), 1055–1064.
Zimmerman, S. M., & Kim, S. K. (2014). The GATA transcription factor/MTA-1 homolog egr-1 promotes longevity and stress resistance in Caenorhabditis elegans. Aging Cell, 13(2), 329–339.
von Zelewsky, T., Palladino, F., Brunschwig, K., Tobler, H., Hajnal, A., & Muller, F. (2000). The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development, 127(24), 5277–5284.
Chen, Z., & Han, M. (2001). Role of C. elegans lin-40 MTA in vulval fate specification and morphogenesis. Development, 128(23), 4911–4921.
Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., et al. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science, 299(5604), 256–259.
Reddy, S. D., Rayala, S. K., Ohshiro, K., Pakala, S. B., Kobori, N., Dash, P., et al. (2011). Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proceedings of the National Academy of Sciences of the United States of America, 108(10), 4200–4205.
Thakur, M. K., & Ghosh, S. (2009). Interaction of estrogen receptor alpha transactivation domain with MTA1 decreases in old mouse brain. Journal of Molecular Neurosciences, 37(3), 269–273.
Aramaki, Y., Ogawa, K., Toh, Y., Ito, T., Akimitsu, N., Hamamoto, H., et al. (2005). Direct interaction between metastasis-associated protein 1 and endophilin 3. FEBS Letters, 579(17), 3731–3736.
Lee, C., Gyorgy, A., Maric, D., Sadri, N., Schneider, R. J., Barker, J. L., et al. (2008). Members of the NuRD chromatin remodeling complex interact with AUF1 in developing cortical neurons. Cerebral Cortex, 18(12), 2909–2919.
Simpson, A., Uitto, J., Rodeck, U., & Mahoney, M. G. (2001). Differential expression and subcellular distribution of the mouse metastasis-associated proteins Mta1 and Mta3. Gene, 273(1), 29–39.
Potts, R. C., Zhang, P., Wurster, A. L., Precht, P., Mughal, M. R., Wood, W. H., 3rd, et al. (2011). CHD5, a brain-specific paralog of Mi2 chromatin remodeling enzymes, regulates expression of neuronal genes. PloS One, 6(9), e24515.
Del Bene, F., Tessmar-Raible, K., & Wittbrodt, J. (2004). Direct interaction of geminin and Six3 in eye development. Nature, 427(6976), 745–749.
Manavathi, B., Peng, S., Rayala, S. K., Talukder, A. H., Wang, M. H., Wang, R. A., et al. (2007). Repression of Six3 by a corepressor regulates rhodopsin expression. Proceedings of the National Academy of Sciences of the United States of America, 104(32), 13128–13133.
Mitton, K. P., Swain, P. K., Chen, S., Xu, S., Zack, D. J., & Swaroop, A. (2000). The leucine zipper of NRL interacts with the CRX homeodomain. A possible mechanism of transcriptional synergy in rhodopsin regulation. Journal of Biological Chemistry, 275(38), 29794–29799.
Qtaishat, N. M., Wiggert, B., & Pepperberg, D. R. (2005). Interphotoreceptor retinoid-binding protein (IRBP) promotes the release of all-trans retinol from the isolated retina following rhodopsin bleaching illumination. Experimental Eye Research, 81(4), 455–463.
Wallis, D. E., Roessler, E., Hehr, U., Nanni, L., Wiltshire, T., Richieri-Costa, A., et al. (1999). Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nature Genetics, 22(2), 196–198.
Li, D. Q., Pakala, S. B., Reddy, S. D., Peng, S., Balasenthil, S., Deng, C. X., et al. (2013). Metastasis-associated protein 1 is an integral component of the circadian molecular machinery. Nature Communication, 4, 2545.
Zhou, B., Ma, Q., Kong, S. W., Hu, Y., Campbell, P. H., McGowan, F. X., et al. (2009). Ackerman KG, Wu B, Zhou B, Tevosian SG, Pu WT.Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. Journal of Clinical Investigation, 119(6), 1462–1676.
Zhang, H., Stephens, L. C., & Kumar, R. (2006). Metastasis tumor antigen family proteins during breast cancer progression and metastasis in a reliable mouse model for human breast cancer. Clinical Cancer Research, 12(5), 1479–1486.
Lagutin, O. V., Zhu, C. C., Kobayashi, D., Topczewski, J., Shimamura, K., Puelles, L., et al. (2003). Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes & Development, 17(3), 368–379.
Kumar, R., Balasenthil, S., Manavathi, B., Rayala, S. K., & Pakala, S. B. (2010). Metastasis-associated protein 1 and its short form variant stimulates Wnt1 transcription through promoting its derepression from Six3 corepressor. Cancer Research, 70(16), 6649–6658.
Zhang, H., Singh, R. R., Talukder, A. H., & Kumar, R. (2006). Metastatic tumor antigen 3 is a direct corepressor of the Wnt4 pathway. Genes & Development, 20(21), 2943–2948.
Li, W., Zhang, J., Liu, X., Xu, R., & Zhang, Y. (2007). Correlation of appearance of metastasis-associated protein1 (Mta1) with spermatogenesis in developing mouse testis. Cell Tissue Research, 329(2), 351–362.
Li, W., Liu, X. P., Xu, R. J., & Zhang, Y. Q. (2007). Immunolocalization assessment of metastasis-associated protein 1 in human and mouse mature testes and its association with spermatogenesis. Asian Journal of Andrology, 9(3), 345–352.
Li, W., Wu, Z. Q., Zhao, J., Guo, S. J., Li, Z., Feng, X., et al. (2011). Transient protection from heat-stress induced apoptotic stimulation by metastasis-associated protein 1 in pachytene spermatocytes. PloS One, 6(10), e26013.
Zhang, S., Li, W., Zhu, C., Wang, X., Li, Z., Zhang, J., et al. (2012). Sertoli cell-specific expression of metastasis-associated protein 2 (MTA2) is required for transcriptional regulation of the follicle-stimulating hormone receptor (FSHR) gene during spermatogenesis. Journal of Biological Chemistry, 287(48), 40471–40483.
Al-Bader, M. D., Kilarkaje, N., El-Farra, A., & Al-Abdallah, A. A. (2014). Expression and subcellular localization of metastasis-associated protein 1, its short form, and estrogen receptors in rat placenta. Reproductive Sciences. doi:10.1177/1933719114549851.
Kwintkiewicz, J., Padilla-Banks, E., Jefferson, W. N., Jacobs, I. M., Wade, P. A., & Williams, C. J. (2012). Metastasis-associated protein 3 (MTA3) regulates G2/M progression in proliferating mouse granulosa cells. Biology of Reproduction, 86(3), 1–8.
Pakala, S. B., Bui-Nguyen, T. M., Reddy, S. D., Li, D. Q., Peng, S., Rayala, S. K., et al. (2010). Regulation of NF-kappaB circuitry by a component of the nucleosome remodeling and deacetylase complex controls inflammatory response homeostasis. Journal of Biological Chemistry, 285(31), 23590–23597.
Pakala, S. B., Reddy, S. D., Bui-Nguyen, T. M., Rangparia, S. S., Bommana, A., & Kumar, R. (2010). MTA1 coregulator regulates LPS response via MyD88-dependent signaling. Journal of Biological Chemistry, 285(43), 32787–32792.
Ghanta, K. S., Pakala, S. B., Reddy, S. D., Li, D. Q., Nair, S. S., & Kumar, R. (2011). MTA1 coregulation of transglutaminase 2 expression and function during inflammatory response. Journal of Biological Chemistry, 286(9), 7132–7138.
Lu, X., Kovalev, G. I., Chang, H., Kallin, E., Knudsen, G., Xia, L., et al. (2008). Inactivation of NuRD component Mta2 causes abnormal T cell activation and lupus-like autoimmune disease in mice. Journal of Biological Chemistry, 283(20), 13825–13833.
Hosokawa, H., Tanaka, T., Suzuki, Y., Iwamura, C., Ohkubo, S., Endoh, K., et al. (2013). Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proceedings of the National Academy of Sciences of the United States of America, 110(12), 4691–4696.
Li, D. Q., Ohshiro, K., Reddy, S. D., Pakala, S. B., Lee, M. H., Zhang, Y., et al. (2009). E3 ubiquitin ligase COP1 regulates the stability and functions of MTA1. Proceedings of the National Academy of Sciences of the United States of America, 106(41), 17493–17498.
Chou, D. M., Adamson, B., Dephoure, N. E., Tan, X., Nottke, A. C., Hurov, K. E., et al. (2010). A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proceedings of the National Academy of Sciences of the United States of America, 107(43), 18475–18480.
Li, D. Q., Divijendra Natha Reddy, S., Pakala, S. B., Wu, X., Zhang, Y., Rayala, S. K., et al. (2009). MTA1 coregulator regulates p53 stability and function. Journal of Biological Chemistry, 284(50), 34545–34552.
Li, D. Q., Pakala, S. B., Reddy, S. D., Ohshiro, K., Peng, S. H., Lian, Y., et al. (2010). Revelation of p53-independent function of MTA1 in DNA damage response via modulation of the p21 WAF1-proliferating cell nuclear antigen pathway. Journal of Biological Chemistry, 285(13), 10044–10052.
Ghanta, K. S., Li, D. Q., Eswaran, J., & Kumar, R. (2011). Gene profiling of MTA1 identifies novel gene targets and functions. PLoS One, 6(2), e17135.
Li, D. Q., Ohshiro, K., Khan, M. N., & Kumar, R. (2010). Requirement of MTA1 in ATR-mediated DNA damage checkpoint function. Journal of Biological Chemistry, 285(26), 19802–19812.
Li, W., Zhu, H., Bao, W., Fu, H., Li, Z., Liu, X., et al. (2008). Involvement of metastasis tumor antigen 1 in hepatic regeneration and proliferation. Cell Physiological Biochemistry, 22(1–4), 315–326.
Nair, S. S., Bommana, A., Pakala, S. B., Ohshiro, K., Lyon, A. J., Suttiprapa, S., et al. (2011). Inflammatory response to liver fluke Opisthorchis viverrini in mice depends on host master coregulator MTA1, a marker for parasite-induced cholangiocarcinoma in humans. Hepatology, 54(4), 1388–1397.
Steiner, L. A., Maksimova, Y., Schulz, V., Wong, C., Raha, D., Mahajan, M. C., et al. (2009). Chromatin architecture and transcription factor binding regulate expression of erythrocyte membrane protein genes. Molecular and Cellular Biology, 29(20), 5399–5412.
Rodriguez, P., Bonte, E., Krijgsveld, J., Kolodziej, K. E., Guyot, B., Heck, A. J., et al. (2005). GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO Journal, 24(13), 2354–2366.
Li, X., Jia, S., Wang, S., Wang, Y., & Meng, A. (2009). Mta3-NuRD complex is a master regulator for initiation of primitive hematopoiesis in vertebrate embryos. Blood, 114(27), 5464–5472.
Kumar, A., Salimath, B. P., Schieker, M., Stark, G. B., & Finkenzeller, G. (2011). Inhibition of metastasis-associated gene 1 expression affects proliferation and osteogenic differentiation of immortalized human mesenchymal stem cells. Cell Proliferation, 44(2), 128–138.
Acknowledgments
We apologize for not citing many other deserving studies from our colleagues due to space limitations. We thank fellows, research staff and colleagues in the Kumar laboratory for their contribution to the biology of MTA1 in cancer. The MTA1 project in Kumar’s lab is supported by NIH grant CA098823.
Conflict of interest
The authors declare no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sen, N., Gui, B. & Kumar, R. Physiological functions of MTA family of proteins. Cancer Metastasis Rev 33, 869–877 (2014). https://doi.org/10.1007/s10555-014-9514-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9514-4