Summary
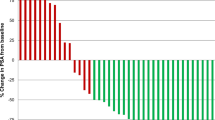
Background SB939 is a potent oral inhibitor of class 1, 2, and 4 histone deacetylases (HDACs). These three HDAC classes are highly expressed in castration resistant prostate cancer (CRPC) and associated with poor clinical outcomes. We designed a phase II study of SB939 in men with metastatic CRPC. Methods Patients received SB939 60 mg on alternate days three times per week for 3 weeks on a 4-week cycle. Primary endpoints were PSA response rate (RR) and progression-free survival (PFS). Secondary endpoints included objective response rate and duration; overall survival; circulating tumor cell (CTC) enumeration and safety. Exploratory correlative studies of the TMPRSS2-ERG fusion and PTEN biomarkers were also performed. Results Thirty-two patients were enrolled of whom 88 % had received no prior chemotherapy. The median number of SB939 cycles administered was three (range 1–8). Adverse events were generally grade 1–2, with five pts experiencing one or more grade three event. One patient died due to myocardial infarction. A confirmed PSA response was noted in two pts (6 %), lasting 3.0 and 21.6 months. In patients with measurable disease there were no objective responses. Six patients had stable disease lasting 1.7 to 8.0 months. CTC response (from ≥5 at baseline to <5 at 6 or 12 weeks) occurred in 9/14 evaluable patients (64 %). Conclusion Although SB939 was tolerable at the dose/schedule given, and showed declines in CTC in the majority of evaluable patients, it did not show sufficient activity based on PSA RR to warrant further study as a single agent in unselected patients with CRPC.



Similar content being viewed by others
References
Abbas A, Gupta S (2008) The role of histone deacetylases in prostate cancer. Epigenetics 3(6):300–309
Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K et al (2008) Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 98(3):604–610
Edwards A, Li J, Atadja P, Bhalla K, Haura EB (2007) Effect of the histone deacetylase inhibitor LBH589 against epidermal growth factor receptor-dependent human lung cancer cells. Mol Cancer Ther 6(9):2515–2524
Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R et al (2007) Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res 13(16):4882–4890
Pratt WB, Galigniana MD, Morishima Y, Murphy PJ (2004) Role of molecular chaperones in steroid receptor action. Essays Biochem 40:41–58
Kantharaj E, Jayaraman R (2011) Histone deacetylase inhibitors as therapeutic agents for cancer therapy: Drug metabolism and pharmacokinetic properties. In: Rundfeldt C, (ed.). Drug development - a case study based insight into modern strategies. InTech, 101
Novotny-Diermayr V, Sangthongpitag K, Hu CY, Wu X, Sausgruber N, Yeo P et al (2010) SB939, a novel potent and orally active histone deacetylase inhibitor with high tumor exposure and efficacy in mouse models of colorectal cancer. Mol Cancer Ther 9(3):642–652
Wang H, Yu N, Chen D, Lee KC, Lye PL, Chang JW et al (2011) Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H-benzimidazol-5-yl}-N-hydroxyacrylami de (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. J Med Chem 54(13):4694–4720
Razak AR, Hotte SJ, Siu LL, Chen EX, Hirte HW, Powers J et al (2011) Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br J Cancer 104(5):756–762
Yong WP, Goh BC, Soo RA, Toh HC, Ethirajulu K, Wood J et al (2011) Phase I and pharmacodynamic study of an orally administered novel inhibitor of histone deacetylases, SB939, in patients with refractory solid malignancies. Ann Oncol 22(11):2516–2522
Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI et al (2006) TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res 66(21):10242–10246
Squire JA (2009) TMPRSS2-ERG and PTEN loss in prostate cancer. Nat Genet 41(5):509–510
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26(7):1148–1159
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H et al (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14(19):6302–6309
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Yoshimoto M, Joshua AM, Cunha IW, Coudry RA, Fonseca FP, Ludkovski O et al (2008) Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol 21(12):1451–1460
Yoshimoto M, Ludkovski O, DeGrace D, Williams JL, Evans A, Sircar K et al (2012) PTEN genomic deletions that characterize aggressive prostate cancer originate close to segmental duplications. Genes Chromosomes Cancer 51(2):149–160
Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA et al (2011) Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol 35(7):1014–1020
Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J et al (2011) PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 17(20):6563–6573
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Welsbie DS, Xu J, Chen Y, Borsu L, Scher HI, Rosen N et al (2009) Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res 69(3):958–966
Molife LR, Attard G, Fong PC, Karavasilis V, Reid AH, Patterson S et al (2010) Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 21(1):109–113
Rathkopf DE, Picus J, Hussain A, Ellard S, Chi KN, Nydam T et al (2013) A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 72(3):537–544
Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J (2007) HDAC inhibitors and cardiac safety. Clin Cancer Res 13(3):1068, author reply -9
Munster P, Marchion D, Bicaku E, Schmitt M, Lee JH, DeConti R et al (2007) Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol 25(15):1979–1985
Munster PN, Marchion D, Thomas S, Egorin M, Minton S, Springett G et al (2009) Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br J Cancer 101(7):1044–1050
Tsai SC, Valkov N, Yang WM, Gump J, Sullivan D, Seto E (2000) Histone deacetylase interacts directly with DNA topoisomerase II. Nat Genet 26(3):349–353
Rathkopf D, Wong BY, Ross RW, Anand A, Tanaka E, Woo MM et al (2010) A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 66(1):181–189
Wissing MD, Mendonca J, Kortenhorst MS, Kaelber NS, Gonzalez M, Kim E et al (2013) Targeting prostate cancer cell lines with polo-like kinase 1 inhibitors as a single agent and in combination with histone deacetylase inhibitors. FASEB J: Off Publ Fed Am Soc Exp Biol 27(10):4279–4293
Ellis L, Ku SY, Ramakrishnan S, Lasorsa E, Azabdaftari G, Godoy A, et al (2013) Combinatorial antitumor effect of HDAC and the PI3K-Akt-mTOR pathway inhibition in a Pten defecient model of prostate cancer. Oncotarget
Acknowledgments
The NCIC Clinical Trials Group is supported by the Canadian Cancer Society Research Institute (grant #021039) l
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
PAB is funded by a Cancer Care Ontario Research Chair in Experimental Therapeutics
Rights and permissions
About this article
Cite this article
Eigl, B.J., North, S., Winquist, E. et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Invest New Drugs 33, 969–976 (2015). https://doi.org/10.1007/s10637-015-0252-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0252-4