Abstract
Purpose
To elucidate the role of the renal basolateral transporter, Oat3, in the disposition of methotrexate.
Materials and Methods
Chinese hamster ovary cells expressing mouse Oat3 were used to determine kinetics and specificity of inhibition of methotrexate transport. Methotrexate clearance was then examined in vivo in wildtype and Oat3 knockout mice.
Results
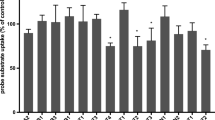
NSAIDs, ß-lactams, and uremic toxins inhibited mOat3-mediated methotrexate uptake by 70–100%, while folate, leucovorin, and 5-methyltetrahydrofolate inhibited transport by 25–50%. A K m of 60.6 ± 9.3 μM for methotrexate transport was determined. Oat3 knockout mice exhibited reduced methotrexate-to-inulin clearance ratios versus wildtype. Male wildtype mice, but not knockouts or females, demonstrated significantly accelerated methotrexate clearance in response to reduced folates. Reduced folates also markedly inhibited hepatic methotrexate accumulation in males, but not females, and the response was independent of Oat3 function.
Conclusions
Oat3 contributes to methotrexate clearance, but represents only one component responsible for methotrexate’s elimination. Therefore, in patients, dysfunctional hOAT3 polymorphisms or drug competition for hOAT3 transport may severely impact methotrexate elimination only when redundant means of methotrexate removal are also compromised. Furthermore, the present findings suggest that reduced-folate administration only influences methotrexate disposition in males, with the renal reduced-folate response influenced by OAT3 function.








Similar content being viewed by others
Abbreviations
- 5-CH3-THF:
-
5-methyltetrahydrofolate
- 5-CHO-THF:
-
5-formyltetrahydrofolate (a.k.a. folinic acid or leucovorin)
- 5-HIAA:
-
5-hydroxyindoleacetic acid
- CHO-FRT:
-
Chinese hamster ovary cells transfected with empty vector
- CHO-mOat3:
-
Chinese hamster ovary cells transfected with murine organic anion transporter 3
- DOPAC:
-
dihydroxyphenylacetic acid
- h:
-
human
- K m :
-
the Michaelis–Menten constant
- m:
-
mouse
- MRP:
-
multidrug resistance-associated protein
- MTX:
-
methotrexate
- NSAIDs:
-
non-steroidal anti-inflammatory drugs
- OAT or Oat:
-
human or non-human organic anion transporter, respectively
- r:
-
rat
- RFC-1:
-
reduced folate carrier 1
References
Methotrexate. DRUGDEX® Evaluations. n.d. Thomson Micromedex. 14 Mar. 2007. Available at: http://www.thomsonhc.com.
B. C. Widemann and P. C. Adamson. Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703 (2006).
J. C. White and I. D. Goldman. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol. Pharmacol. 12:711–719 (1976).
J. C. White, S. Loftfield, and I. D. Goldman. The mechanism of action of methotrexate. III. Requirement of free intracellular methotrexate for maximal suppression of (14C)formate incorporation into nucleic acids and protein. Mol. Pharmacol. 11:287–297 (1975).
S. H. Wright and W. H. Dantzler. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 84:987–1049 (2004).
D. H. Sweet, K. T. Bush, and S. K. Nigam. The organic anion transporter family: from physiology to ontogeny and the clinic. Am. J. Physiol. Renal. Physiol. 281:F197–205 (2001).
D. H. Sweet. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol. Appl. Pharmacol. 204:198–215 (2005).
A. A. El-Sheikh, J. J. van den Heuvel, J. B. Koenderink, and F. G. Russel. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 320:229–235 (2007).
M. Kool, M. van der Linden, M. de Haas, G. L. Scheffer, J. M. de Vree, A. J. Smith, G. Jansen, G. J. Peters, N. Ponne, R. J. Scheper, R. P. Elferink, F. Baas, and P. Borst. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc. Natl. Acad. Sci. U. S. A. 96:6914–6919 (1999).
T. Rau, B. Erney, R. Gores, T. Eschenhagen, J. Beck, and T. Langer. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin. Pharmacol. Ther. 80:468–476 (2006).
I. J. Letourneau, R. J. Bowers, R. G. Deeley, and S. P. Cole. Limited modulation of the transport activity of the human multidrug resistance proteins MRP1, MRP2 and MRP3 by nicotine glucuronide metabolites. Toxicol. Lett. 157:9–19 (2005).
K. E. Brigle, M. J. Spinella, E. E. Sierra, and I. D. Goldman. Characterization of a mutation in the reduced folate carrier in a transport defective L1210 murine leukemia cell line. J. Biol. Chem. 270:22974–22979 (1995).
Y. Nozaki, H. Kusuhara, H. Endou, and Y. Sugiyama. Quantitative evaluation of the drug–drug interactions between methotrexate and nonsteroidal anti-inflammatory drugs in the renal uptake process based on the contribution of organic anion transporters and reduced folate carrier. J. Pharmacol. Exp. Ther. 309:226–234 (2004).
I. Badagnani, R. A. Castro, T. R. Taylor, C. M. Brett, C. C. Huang, D. Stryke, M. Kawamoto, S. J. Johns, T. E. Ferrin, E. J. Carlson, E. G. Burchard, and K. M. Giacomini. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J. Pharmacol. Exp. Ther. 318:521–529 (2006).
H. Saito, S. Masuda, and K. Inui. Cloning and functional characterization of a novel rat organic anion transporter mediating basolateral uptake of methotrexate in the kidney. J. Biol. Chem. 271:20719–20725 (1996).
A. Takeuchi, S. Masuda, H. Saito, T. Abe, and K. Inui. Multispecific substrate recognition of kidney-specific organic anion transporters OAT-K1 and OAT-K2. J. Pharmacol. Exp. Ther. 299:261–267 (2001).
D. H. Sweet, N. A. Wolff, and J. B. Pritchard. Expression cloning and characterization of rOat1. The basolateral organic anion transporter in rat kidney. J. Biol. Chem. 272:30088–30095 (1997).
D. H. Sweet, L. M. Chan, R. Walden, X. P. Yang, D. S. Miller, and J. B. Pritchard. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am. J. Physiol. Renal. Physiol. 284:F763–769 (2003).
T. L. Witt, S. E. Stapels, and L. H. Matherly. Restoration of transport activity by co-expression of human reduced folate carrier half-molecules in transport-impaired K562 cells: localization of a substrate binding domain to transmembrane domains 7–12. J. Biol. Chem. 279:46755–46763 (2004).
Y. L. He, Y. Tanigawara, M. Yasuhara, and R. Hori. Effect of folinic acid on tissue residence and excretion of methotrexate in rats. Drug Metab. Dispos. 19:729–734 (1991).
A. R. Erdman, L. M. Mangravite, T. J. Urban, L. L. Lagpacan, R. A. Castro, M. de la Cruz, W. Chan, C. C. Huang, S. J. Johns, M. Kawamoto, D. Stryke, T. R. Taylor, E. J. Carlson, T. E. Ferrin, C. M. Brett, E. G. Burchard, and K. M. Giacomini. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am. J. Physiol. Renal. Physiol. 290:F905–912 (2006).
K. P. Brady, H. Dushkin, D. Fornzler, T. Koike, F. Magner, H. Her, S. Gullans, G. V. Segre, R. M. Green, and D. R. Beier. A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics 56:254–261 (1999).
G. W. Schnabolk, G. L. Youngblood, and D. H. Sweet. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20). Am. J. Physiol. Renal. Physiol. 291:F314–321 (2006).
Y. Uwai, R. Taniguchi, H. Motohashi, H. Saito, M. Okuda, and K. Inui. Methotrexate–loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab. Pharmacokinet. 19:369–374 (2004).
M. Takeda, S. Khamdang, S. Narikawa, H. Kimura, M. Hosoyamada, S. H. Cha, T. Sekine, and H. Endou. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J. Pharmacol. Exp. Ther. 302:666–671 (2002).
C. Kneuer, K. U. Honscha, and W. Honscha. Rat reduced-folate carrier-1 is localized basolaterally in MDCK kidney epithelial cells and contributes to the secretory transport of methotrexate and fluoresceinated methotrexate. Cell Tissue Res. 320:517–524 (2005).
B. C. Burckhardt and G. Burckhardt. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev. Physiol. Biochem. Pharmacol. 146:95–158 (2003).
A. Takeuchi, S. Masuda, H. Saito, T. Doi, and K. Inui. Role of kidney-specific organic anion transporters in the urinary excretion of methotrexate. Kidney Int. 60:1058–1068 (2001).
D. Sykes, D. H. Sweet, S. Lowes, S. K. Nigam, J. B. Pritchard, and D. S. Miller. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am. J. Physiol. Renal. Physiol. 286:F972–978 (2004).
D. H. Sweet, D. S. Miller, J. B. Pritchard, Y. Fujiwara, D. R. Beier, and S. K. Nigam. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J. Biol. Chem. 277:26934–26943 (2002).
S. C. Buist and C. D. Klaassen. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab. Dispos. 32:620–625 (2004).
S. H. Cha, T. Sekine, J. I. Fukushima, Y. Kanai, Y. Kobayashi, T. Goya, and H. Endou. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol. Pharmacol. 59:1277–1286 (2001).
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-067216 (D.H.S.), by fellowship provisions from the American Foundation for Pharmaceutical Education (A.L.V.), and by the National Institutes of Health Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
VanWert, A.L., Sweet, D.H. Impaired Clearance of Methotrexate in Organic Anion Transporter 3 (Slc22a8) Knockout Mice: A Gender Specific Impact of Reduced Folates. Pharm Res 25, 453–462 (2008). https://doi.org/10.1007/s11095-007-9407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9407-0