Abstract
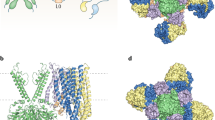
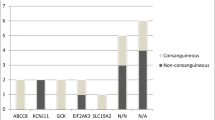
The ATP-sensitive potassium (KATP) channel is composed of two subunits SUR1 and Kir6.2. The channel is key for glucose stimulated insulin release from the pancreatic beta cell. Activating mutations have been identified in the genes encoding these subunits, ABCC8 and KCNJ11, and account for approximately 40% of permanent neonatal diabetes cases. The majority of patients with a KATP mutation present with isolated diabetes however some have presented with the Developmental delay, Epilepsy and Neonatal Diabetes syndrome. This review focuses on mutations in the KATP channel which result in permanent neonatal diabetes, we review the clinical and functional effects as well as the implications for treatment.



Similar content being viewed by others
References
Iafusco D et al. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002;456:798–804.
Edghill EL et al. HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes. 2006;556:1895–8.
Gardner RJ et al. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet. 2000;94:589–96.
Flanagan SE et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;567:1930–7.
Wiedemann B et al. Incidence of neonatal diabetes in Austria-calculation based on the Austrian Diabetes Register. Pediatr Diabetes. 2010;111:18–23.
Slingerland AS et al. Referral rates for diagnostic testing support an incidence of permanent neonatal diabetes in three European countries of at least 1 in 260,000 live births. Diabetologia. 2009;528:1683–5.
Stanik J et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007;924:1276–82.
Greeley SA et al. Update in neonatal diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;171:13–9.
Sellick GS et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;3612:1301–5.
Senee V et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;386:682–7.
Rubio-Cabezas O, et al. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59:2326–31.
Stoffers DA et al. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;151:106–10.
Smith SB, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–80.
Stoy J et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;10438:15040–4.
Edghill EL et al. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;574:1034–42.
Garin I et al. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proc Natl Acad Sci USA. 2010;1077:3105–10.
Ashcroft FM et al. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55(Suppl):54–65.
Thomas PM et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;2685209:426–9.
Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;511:1809–12.
Koster JC et al. Targeted overactivity of beta cell KATP channels induces profound neonatal diabetes. Cell. 2000;1006:645–54.
Gloyn AL et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;35018:1838–49.
Proks P et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 ABCC8 causes neonatal diabetes. Hum Mol Genet. 2006;1511:1793–800.
Babenko AP et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;3555:456–66.
Remedi MS, Koster JC. KATP channelopathies in the pancreas. Pflugers Arch. 2010;460:307–20.
Flanagan SE et al. Update of mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 KCNJ11 and sulfonylurea receptor 1 ABCC8 in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;302:170–80.
Craig TJ, et al. An in-frame deletion in Kir6.2 KCNJ11 causing neonatal diabetes reveals a site of interaction between Kir6.2 and SUR1. J Clin Endocrinol Metab. 2009;26:2551–7.
Ellard S et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;812:375–82.
Sagen JV et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;5310:2713–8.
Vaxillaire M et al. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004;5310:2719–22.
Massa O et al. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum Mutat. 2005;251:22–7.
Flanagan SE et al. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;496:1190–7.
Gloyn AL et al. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 Gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. J Clin Endocrinol Metab. 2004;898:3932–5.
Edghill EL et al. Origin of de novo KCNJ11 mutations and risk of neonatal diabetes for subsequent siblings. J Clin Endocrinol Metab. 2007;925:1773–7.
Pearson ER et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;3555:467–77.
Mohamadi A, et al. Medical and developmental impact of transition from subcutaneous insulin to oral glyburide in a 15-yr-old boy with neonatal diabetes mellitus and intermediate DEND syndrome: extending the age of KCNJ11 mutation testing in neonatal DM. Pediatr Diabetes. 2010;11:203-11.
Masia R et al. An ATP-binding mutation G334D in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;562:328–36.
Sakura H et al. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;3773:338–44.
Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;549:2503–13.
Patch AM et al. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007;9 Suppl 2:28–39.
Shimomura K et al. Mutations at the same residue R50 of Kir6.2 KCNJ11 that cause neonatal diabetes produce different functional effects. Diabetes. 2006;556:1705–12.
Tammaro P et al. A Kir6.2 mutation causing severe functional effects in vitro produces neonatal diabetes without the expected neurological complications. Diabetologia. 2008;515:802–10.
Shimomura K et al. Adjacent mutations in the gating loop of Kir6.2 produce neonatal diabetes and hyperinsulinism. EMBO. Mol Med. 2009;13:166–77.
Proks P et al. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA. 2004;10150:17539–44.
Proks P et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;65:470–5.
Zingman LV et al. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem. 2002;27716:14206–10.
Aittoniemi J et al. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009;3641514:257–67.
de Wet H et al. Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc Natl Acad Sci USA. 2007;10448:18988–92.
de Wet H et al. A mutation R826W in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep. 2008;97:648–54.
Proks P et al. Mechanism of action of a sulphonylurea receptor SUR1 mutation F132L that causes DEND syndrome. Hum Mol Genet. 2007;1616:2011–9.
Girard CA et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;1191:80–90.
Clark RH, et al. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329:458–61.
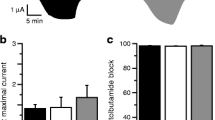
Rafiq M et al. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 SUR1 mutations. Diab Care. 2008;312:204–9.
Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;467:875–91.
Codner E et al. High-dose glibenclamide can replace insulin therapy despite transitory diarrhea in early-onset diabetes caused by a novel R201L Kir6.2 mutation. Diab Care. 2005;283:758–9.
Kumaraguru J et al. Tooth discoloration in patients with neonatal diabetes after transfer onto glibenclamide: a previously unreported side effect. Diab Care. 2009;328:1428–30.
Zung A et al. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;8911:5504–7.
Slingerland AS et al. Improved motor development and good long-term glycaemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia. 2006;4911:2559–63.
Slingerland AS et al. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med. 2008;253:277–81.
Shimomura K et al. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007;6913:1342–9.
Gurgel LC et al. Sulfonylrea treatment in permanent neonatal diabetes due to G53D mutation in the KCNJ11 gene: improvement in glycemic control and neurological function. Diab Care. 2007;3011:e108.
Acknowledgements
We thank Professor Frances Ashcroft for her contribution to Fig. 3. SE is a core member of the Peninsula NIHR Clinical Research Facility. EE is supported by the European Union Integrated Project EURODIA (LSHM-CT-2006-518153 in the Framework Programme 6 of the European-Community). SEF is the Peninsula College of Medicine and Dentistry Sir Graham Wilkins Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edghill, E.L., Flanagan, S.E. & Ellard, S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11 . Rev Endocr Metab Disord 11, 193–198 (2010). https://doi.org/10.1007/s11154-010-9149-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-010-9149-x