Abstract
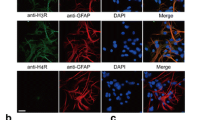
Histamine is a potent mediator of inflammation and a regulator of innate and adaptive immune responses. However, the influence of histamine on microglia, the resident immune cells in the brain, remains uninvestigated. In the present study, we found that microglia can constitutively express all four histamine receptors (H1R, H2R, H3R, and H4R), and the expression of H1R and H4R can be selectively upregulated in primary cultured microglia in a dose-dependent manner by histamine. Histamine can also dose-dependently stimulate microglia activation and subsequently production of proinflammatory factors tumor necrosis factor (TNF)-alpha and interleukin-6 (IL-6). The antagonists of H1R and H4R but not H2R and H3R reduced histamine-induced TNF-alpha and IL-6 production, MAPK and PI3K/AKT pathway activation, and mitochondrial membrane potential loss in microglia, suggesting that the actions of histamine are via H1R and H4R. On the other hand, inhibitors of JNK, p38, or PI3K suppressed histamine-induced TNF-alpha and IL-6 release from microglia. Histamine also activated NF-kappa B and ammonium pyrrolidinedithiocarbamate, an inhibitor of NF-kappa B, and reduced histamine-induced TNF-alpha and IL-6 release. In summary, the present study identifies the expression of histamine receptors on microglia. We also demonstrate that histamine induced TNF-alpha and IL-6 release from activated microglia via H1R and H4R-MAPK and PI3K/AKT-NF-kappa B signaling pathway, which will deepen the understanding of microglia-mediated neuroinflammatory symptoms of chronic neurodegenerative disease.








Similar content being viewed by others
References
Csuka E, Hans VH, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC (2000) Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport 11:2587–2590
Popovich PG (2000) Immunological regulation of neuronal degeneration and regeneration in the injured spinal cord. Prog Brain Res 128:43–58
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci 24:395–401
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152
Block ML, Hong JS (2005) Microglial and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98
Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A (2005) Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm 112:111–119
Parsons ME, Ganellin CR (2006) Histamine and its receptors. Br J Pharmacol 47(Suppl 1):127–135
Akdis CA, Simons FE (2006) Histamine receptors are hot in immunopharmacology. Eur J Pharmacol 533:69–76
Repka-Ramirez MS, Baraniuk JN (2002) Histamine in health and disease. Clinical Allergy and Immunology 17:1–25
Tiligada E, Zampeli E, Sander K, Stark H (2009) Histamine H3 and H4 receptors as novel drug targets. Expert Opinion on Investigating Drugs 18:1519–1531
Huang JF, Thurmond RL (2008) The new biology of histamine receptors. Current Allergy and Asthma Reports 8:21–27
Ferreira R, Santos T, Gonçalves J, Baltazar G, Ferreira L, Agasse F, Bernardino L (2012) Histamine modulates microglia function. J Neuroinflammation 9:90
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglial in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL (2005) Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 51:199–208
Ciallella JR, Saporito M, Lund S, Leist M, Hasseldam H, McGann N, Smith CS, Bozyczko-Coyne D, Flood DG (2005) CEP-11004, an inhibitor of the SAPK/JNK pathway, reduces TNF-alpha release from lipopolysaccharide-treated cells and mice. Eur J Pharmacol 515:179–187
Lund S, Porzgen P, Mortensen AL, Hasseldam H, Bozyczko-Coyne D, Morath S, Hartung T, Bianchi M, Ghezzi P, Bsibsi M, Dijkstra S, Leist M (2005) Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. J Neurochem 92:1439–1451
Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK (2005) c-Jun N-ter-minal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50:235–246
Desai P, Thurmond RL (2011) Histamine H4 receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur J Immunol 41:1764–1773
Bonaiuto C, McDonald PP, Rossi F, Cassatella MA (1997) Activation of nuclear factor-kappa B by beta-amyloid peptides and interferon-gamma in murine microglia. J Neuroimmunol 77:51–56
Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJ, Anthony DC (2011) The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation 8:166
Carmody RJ, Chen YH (2007) Nuclear factor-kappaB: activation and regulation during toll-like receptor signaling. Cell Mol Immunol 4:31–41
Kim BW, Koppula S, Kim IS, Lim HW, Hong SM, Han SD, Hwang BY, Choi DK (2011) Antineuroinflammatory activity of Kamebakaurin from Isodon japonicus via inhibition of c-Jun NH-terminal kinase and p38 mitogen-activated protein kinase pathway in activated microglial cells. J Pharmacol Sci 116:296–308
Minghetti L (2005) Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 18:315–321
Russel WL, Henry DP, Phebus LA, Clemens JA (1990) Release of histamine in rat hypothalamus and corpus striatum in vivo. Brain Res 512:95–101
Ikarashi Y, Yuzurihara M (2002) Experimental anxiety induced by histaminergics in mast cell-deficient and congenitally normal mice. Pharmacol Biochem Behav 72:437–441
Vizuete ML, Merino M, Venero JL, Santiago M, Cano J, Machado A (2000) Histamine infusion induces a selective dopaminergic neuronal death along with an inflammatory reaction in rat substantia nigra. J Neurochem 75:540–552
Wei J, Wu F, Sun X, Zeng X, Liang JY, Zheng HQ, Yu XB, Zhang KX, Wu ZD (2013) Differences in microglia activation between rats-derived cell and mice-derived cell after stimulating by soluble antigen of IV larva from Angiostrongylus cantonensis in vitro. Parasitol Res 112:207–214
Lee JY, Jhun BS, Oh YT, Lee JH, Choe W, Baik HH, Ha J, Yoon KS, Kim SS, Kang I (2006) Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-α production through inhibition of PI3-kinase/Akt and NF-κB activation in murine BV2 microglial cells. Neurosci Lett 396:1–6
Oh YT, Lee JY, Lee J, Kim H, Yoon KS, Choe W, Kang I (2009) Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-kappaB signaling pathways. Neurosci Lett 464:93–97
Jana M, Jana A, Liu X, Ghosh S, Pahan K (2007) Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of IκBα in anti-inflammatory effect of gemfibrozil in microglia. J Immunol 179:4142–4152
Medina MA, Quesada AR, de Castro I, Sánchez-Jiménez F (1999) Histamine, polyamines and cancer. Biochem Pharmacol 57:1341–1344
Saligrama N, Noubade R, Case LK, del Rio R, Teuscher C (2012) Combinatorial roles for histamine H1-H2 and H3-H4 receptors in autoimmune inflammatory disease of the central nervous system. Eur J Immunol 42:1536–1546
Johnson D, Yasui D, Seeldayers P (1991) An analysis of mast cell frequency in the rodent nervous system: numbers vary between different strains and can be reconstituted in mast cell-deficient mice. J Neuropathol Exp Neurol 50:227–234
Jin Y, Silverman AJ, Vannucci SJ (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 40:3107–3112
Galli SJ, Nakae S, Tsai M (2005) Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142
Feuser K, Thon KP, Bischoff SC, Lorentz A (2012) Human intestinal mast cells are a potent source of multiple chemokines. Cytokine 58:178–185
Bulanova E, Bulfone-Paus S (2010) P2 receptor-mediated signaling in mast cell biology. Purinergic Signal 6:3–17
Juremalm M, Hjertson M, Olsson N, Harvima I, Nilsson K, Nilsson G (2000) The chemokine receptor CXCR4 is expressed within the mast cell lineage and its ligand stromal cell-derived factor-1alpha acts as a mast cell chemotaxin. Eur J Immunol 30:3614–3622
Skuljec J, Sun H, Pul R, Bénardais K, Ragancokova D, Moharregh-Khiabani D, Kotsiari A, Trebst C, Stangel M (2011) CCL5 induces a pro-inflammatory profile in microglia in vitro. Cell Immunol 270:164–171
Chakraborty S, Kaushik DK, Gupta M, Basu A (2010) Inflammasome signaling at the heart of central nervous system pathology. J Neurosci Res 88:1615–1631
Wang X, Li C, Chen Y, Hao Y, Zhou W, Chen C, Yu Z (2008) Hypoxia enhances CXCR4 expression favoring microglia migration via HIF-1 activation. Biochem. Biophys Res Commun 371:283–288
Zhang S, Zeng X, Yang H, Hu G, He S (2012) Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem 29:931–940
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A (2008) Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol 18:1–9
Skaper SD, Facci L (2012) Mast cell–glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Phil Trans R Soc B 367:3312–3325
Acknowledgment
This project was sponsored by the grants from the National Natural Science Foundation of China (no. 81102422, 81373398), the Natural Science Foundation of Jiangsu Province (BK2010020), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest
The authors declare that this article content has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hongquan Dong and Wei Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, H., Zhang, W., Zeng, X. et al. Histamine Induces Upregulated Expression of Histamine Receptors and Increases Release of Inflammatory Mediators from Microglia. Mol Neurobiol 49, 1487–1500 (2014). https://doi.org/10.1007/s12035-014-8697-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8697-6