Abstract
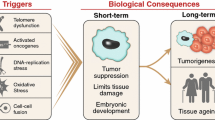
Depending on the cell type and tissue environment, epithelial and mesenchymal cell phenotypes are not static and can be highly dynamic. Epithelial-mesenchymal transitions (EMTs) and reverse EMTs provide flexibility during embryogenesis. While EMTs are a critical normal process during development and wound healing, properties of the EMT have been implicated in human pathology, particularly cancer metastasis. A normal undamaged epithelium does not typically exhibit features of an EMT. However, particularly under the influence of the surrounding microenvironment, cancer cells may reactivate developmental phenotypes out of context in the adult. This reactivation, such as the EMT, can facilitate tumor cell invasion and metastasis, and therefore is a major mechanism of tumor progression. Conversely, cellular senescence, which is associated with aging, is a process by which cells enter a state of permanent cell cycle arrest, thereby constituting a potent tumor suppressive mechanism. However, accumulating evidence shows that senescent cells can have deleterious effects on the tissue microenvironment. The most significant of these effects is the acquisition of a senescence-associated secretory phenotype (SASP) that turns senescent fibroblasts into pro-inflammatory cells having the ability to promote tumor progression, in part by inducing an EMT in nearby epithelial cells. Here, we summarize the potential impacts of SASP factors, particularly interleukins, on tissue microenvironments and their ability to stimulate tumor progression through induction of an EMT.

Similar content being viewed by others
References
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428
Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15(2):117–134
Peinado H, Portillo F, Cano A (2004) Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol 48(5–6):365–375
Birchmeier W, Behrens J (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1198(1):11–26
Prasad CP et al (2009) Expression analysis of E-cadherin, Slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer 9:325
Logullo AF et al (2010) Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma: association with progression. Oncol Rep 23(2):313–320
Mori M et al (2009) Zyxin mediates actin fiber reorganization in epithelial-mesenchymal transition and contributes to endocardial morphogenesis. Mol Biol Cell 20(13):3115–3124
Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5(15):1597–1601
Olumi AF et al (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59:5002–5011
Erez N et al (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147
Qiu W et al (2008) No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet 40(5):650–655
Rodier F et al (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11(8):973–979
Gilbert LA, Hemann MT (2010) DNA damage-mediated induction of a chemoresistant niche. Cell 143(3):355–366
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
d’Adda di Fagagna F et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nature Rev Molec Cell Biol 8:729–740
Gorgoulis VG, Halazonetis TD (2010) Oncogene-induced senescence: the bright and dark side of the response. Curr Opin Cell Biol 22(6):816–827
Prieur A, Peeper DS (2008) Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol 20:150–155
Dimri GP (2005) What has senescence got to do with cancer? Cancer Cell 7:505–512
Coppe JP et al (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Coppe JP et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6(12):2853–2868
Campisi J (2005) Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell 120:513–522
Dimri GP et al (1995) A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
Jeyapalan JC et al (2007) Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128:36–44
Paradis V et al (2001) Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol 32:327–332
Erusalimsky JD, Kurz DJ (2005) Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol 40(8–9):634–642
Martin JA, Buckwalter JA (2003) The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am 85:106–110
Roberts S et al (2006) Senescence in human intervertebral discs. Eur Spine J 15:312–316
Finch CE, Crimmins EM (2004) Inflammatory exposure and historical changes in human life-spans. Science 305:1736–1739
Parrinello S et al (2005) Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 118(Pt 3):485–496
Freund A et al (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16(5):238–246
Young AR, Narita M (2009) SASP reflects senescence. EMBO Rep 10(3):228–230
Krtolica A et al (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98:12072–12077
Liu D, Hornsby PJ (2007) Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res 67:3117–3126
Coppe JP et al (2010) A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One 5(2):e9188
Bavik C et al (2006) The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res 66:794–802
Wang B et al (2006) A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res 66:3071–3077
Tsai KK et al (2005) Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res 65:6734–6744
Ohuchida K et al (2004) Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res 64(9):3215–3222
Potempa S, Ridley AJ (1998) Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell 9(8):2185–2200
Paumelle R et al (2002) Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 21(15):2309–2319
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454
Tonini T, Rossi F, Claudio PP (2003) Molecular basis of angiogenesis and cancer. Oncogene 22(42):6549–6556
Birchmeier C et al (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12):915–925
Yuan A et al (2005) The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci 10:853–865
Badache A, Hynes NE (2001) Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res 61:383–391
Camphausen K et al (2001) Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res 61(5):2207–2211
Qian LW et al (2002) Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res 8(4):1223–1227
Coppe JP et al (2008) A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol Cancer Res 6(7):1085–1098
Coppe JP et al (2006) Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem 281(40):29568–29574
Strieter RM et al (2006) Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer 42(6):768–778
Orr FW, Wang HH (2001) Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg Oncol Clin N Am 10(2):357–381, ix-x
Nickoloff BJ et al (2004) Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res 64(9):2956–2961
Mantovani A (2004) Chemokines in neoplastic progression. Semin Cancer Biol 14(3):147–148
Xue W et al (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–650
Homey B, Muller A, Zlotnik A (2002) Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol 2(3):175–184
Balkwill F (2004) Cancer and the chemokine network. Nat Rev Cancer 4(7):540–550
Ben-Baruch A (2006) Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol 16(1):38–52
Cowin P, Rowlands TM, Hatsell SJ (2005) Cadherins and catenins in breast cancer. Curr Opin Cell Biol 17:499–508
Kokkinos MI et al (2007) Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs 185:191–203
Dhawan P et al (2005) Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest 115:1765–1776
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Bissell MJ, Radisky D (2001) Putting tumours in context. Nature Rev Cancer 1:46–54
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Brabletz T et al (2005) Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs 179(1–2):56–65
Acknowledgments
The authors wish to thank Dr. Andrew P. Smith for editing the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laberge, RM., Awad, P., Campisi, J. et al. Epithelial-Mesenchymal Transition Induced by Senescent Fibroblasts. Cancer Microenvironment 5, 39–44 (2012). https://doi.org/10.1007/s12307-011-0069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-011-0069-4