Abstract
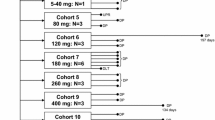
Objectives: CI-994 (N-acetyl dinaline, PD123654) is a novel oral agent active in a broad variety ofmurine and human tumor xenografts. While cytotoxic in theBrown Norway (BN) rat leukemia model, growth inhibition inother murine and human tumor xenografts is predominantlycytostatic. Its specific mechanism of action remains unknown.Following CI-994 administration, inhibition of both histonedeacetylation and cellular proliferation at the G1 to Stransition phase of the cell cycle are observed. This Phase 1study in patients with solid tumors was carried out todetermine a maximum tolerated daily oral dose (MTD) for CI-994administered on a chronic basis. Methods: Fifty-threepatients received CI-994 daily for treatment durations rangingfrom 2 to 10 weeks. Dosage escalation proceeded in 2 phases;an Acute Dosing Phase (n = 11) to define the MTD for CI-994administered over 2 weeks and a Chronic Dosing Phase (n = 29)to define the MTD for daily administration for 8 weeks. Uponcompletion of the Chronic Dosing Phase, a third cohort ofpatients (n = 13) received CI-994 at the recommended Phase 2dose and schedule with 2 additional single doses of drugadministered separated by a 1-week washout to assess theeffect of food on CI-994 pharmacokinetics. Results:Thrombocytopenia was dose limiting at the MTD of 8mg/m2/day for 8 weeks. Other toxicities includedfatigue and gastrointestinal effects such as nausea, vomiting,diarrhea, constipation and mucositis. Pharmacokinetic studiesrevealed that peak plasma levels and AUC's generally increasedwith dose and that food intake did not affect the rate orextent of drug absorption. One patient with heavilypre-treated adenocarcinoma of the lung achieved a PartialResponse (PR) lasting over 2 years and 3 additional patientsachieved Stable Disease (SD), 1 each with non-small cell lung,colorectal, and renal cancer. Conclusions: Therecommended Phase 2 starting dose is 8 mg/m2/dayfor 8 weeks repeated after a 2-week drug-freeinterval.
Similar content being viewed by others
References
Berger MR, Bischoff H, Fritschi E, Henne T, Hermann M, Pool BL, Satzinger G, Schmahl D, Weierschuasen U: Synthesis, toxicity and therapeutic efficacy of 4–amino-N-2–aminophenyl-benzamide: a new compound preferentially active in slowly growing tumors. Cancer Treat Rep 69: 1415–1424, 1985
Lelieveld P, Middeldorp RJF, Van Putten LM: Effectiveness of P-aminobenzoyl-O-phenylenediamine (GOE 1734) against mouse, rat and human tumor cells. Cancer Chemother Pharmacol 15: 88–90, 1985
Berger MR, Schmahl D: Antineoplastic activity of two 4–amino-N-2–aminophenylbenzamide (GOE 1734) derivatives in rat mammary carcinoma. (Abstr) Proc Am Assoc Cancer Res 28: 301, 1987
Berger MR, Richter H, Seelig MH, Eibl H, Schmahl D: New cytostatics: more activity and less toxicity. Cancer Treat Rev 17: 143–154, 1990
Kraker AJ, Moore CW: Activity and effects of 4–amino-N-2–aminophenylbenzamide (GOE1734, PD 104208): a compound with a novel mechanism of action, against HCT-8. (Abstr) Proc Am Ass Cancer Res 30: 584, 1991
Hagenbeek A, Weiershausen U, Martens ACM: Dinaline: a new oral drug against leukemia? Preclinical studies in a relevant rat model for human acute myelocytic leukemia. Leukemia (Baltimore) 2: 226–230, 1988
LoRusso PM, Demchik L, Foster B, Knight J, Bissery M, Polin LM, Leopold WR, Corbett TH: Preclinical antitumor activity of CI-994. Invest New Drugs 14: 349–356, 1996
Leopold WR, Hook KE, Fry DW: Activity and biochemical properties of GOE 1734 (PD 104208), and anticancer agent with a novel mechanism of activity. (Abstr) Proc Am Ass Cancer Res 28: 302, 1987
Rummel SA, Kraker AJ, Steinkampf RW, Hook KE, Klohs WD: Role of a small molecular weight phosphoprotein in the mechanism of action of CI-994 (N-acetyldinaline). Int J Cancer 62: 636–642, 1995
Klohs W, Kraker A, Steinkampf R, Moore C, Dominick M: Mitochondrial involvement in the mechanism of action of the novel antitumor compound 4–(acetylamino)-N-(2–aminophenyl) benzamide. (Abstr) Proc Amer Assoc Cancer Res 32: 396, 1991
Kraker AJ, Hartl BG, Miin J, Merriman RL: Effect of CI-994 on histone acetylation and differentiation in HCT-8 colon carcinoma cells. (Abstr) Proc Amer Assoc Cancer Res 40: 809, 1999
Graziano MJ, Pilcher GD, Walsh KM, Kasali OB, Radulovic L: Preclinical toxicity of a new oral anticancer drug, CI-994 (acetyldinaline), in rats and dogs. Invest New Drugs 15: 295–310, 1997
Foster BJ, Jones L, Weigand R, LoRusso PM, Corbett TH: Preclinical pharmacokinetic, antitumor and toxicity studies with CI-994 (N-acetyldinaline). Invest New Drugs 15: 187–194, 1997
Mehta C, Patel N: StatXact: Statistical software for Exact Nonparametric Inference, Version 3.0. Cambridge, MA: Cytel Software Corporation, 1996
Corbett TH, Roberts BJ, Leopold WR et al.: Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BI/6 mice. Cancer Res 44: 717–726, 1984
Howard CT, Roberts BJ, Vincent PW, Elliott WL: NAcetyldinaline - a small molecule with in vivo cytostatic activity. (Abstr) Proc Am Assoc Cancer Res 33: 393, 1992
Howard CT, Guisbert EZ, Schaefer J, Merriman RL: In vivo antitumor activity of CI-994 (N-acetyl-dinaline, GOE 5549) alone and in combination with gemcitabine against the LC-12 squamous cell lung carcinoma. (Abstr) Proc Am Assoc Cancer Res 40: A3893, 1999 Address for offprints: Patricia M. LoRusso, Department of Hematology and Oncology, Harper Hospital, 5 Hudson, 3990 John R, Detroit, MI 48201, USA; Fax: (313) 993–0559.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prakash, S., Foster, B.J., Meyer, M. et al. Chronic Oral Administration of CI-994: A Phase I Study. Invest New Drugs 19, 1–11 (2001). https://doi.org/10.1023/A:1006489328324
Issue Date:
DOI: https://doi.org/10.1023/A:1006489328324