Abstract
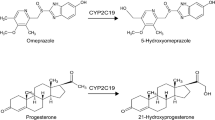
The catalytic requirements and the role of P450 3A9, a female-specific isoform of CYP3A from rat brain, in the metabolism of several steroid hormones were studied using recombinant P450 3A9 protein. The optimal steroid hormone hydroxylase activities of P450 3A9 required cholate but not cytochrome b5. P450 3A9 was active in the hydroxylation reactions of testosterone, androstenedione, progesterone and dehydroepiandrosterone (DHEA). No activity of P450 3A9 toward cortisol was detectable under our reconstitution conditions. Among all the steroid hormones examined, female-specific P450 3A9 seemed to catalyze most efficiently the metabolism of progesterone, one of the major female hormones, to form three mono-hydroxylated products, 6β-, 16α-, and 21-hydroxyprogesterone. Our data also showed that P450 3A9 can catalyze the formation of a dihydroxy product, 4-pregnen-6β, 21-diol-3, 20-dione, from progesterone with a turnover number, 1.3 nmol/min/nmol P450. Based on the Vmax/Km values for P450 3A9 using either 21-hydroxprogesterone or 6β-hydroxyprogesterone as a substrate, 4-pregnen-6β, 21-diol-3, 20-dione may be formed either by 6β-hydroxylation of 21-hydroxprogesterone or 21-hydroxylation of 6β-hydroxyprogesterone. As a major isoform of CYP3A expressed in rat brain, the activities of P450 3A9 toward two major neurosteroids, progesterone and DHEA suggested a possible role for P450 3A9 in the metabolism of neurosteroids.
Similar content being viewed by others
References
Waxman DJ, Dannan GA, Guengerich FP: Regulation of rat hepatic cytochrome P-450: Age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry 24: 4409-4417, 1985
Ciaccio PJ, Halpert JR: Characterization of a phenobarbital-inducible dog liver cytochrome P450 structurally related to rat and human enzymes of the P450IIIA (steroid-inducible) gene subfamily. Arch Biochem Biophys 271: 284-299, 1989
Brian WR, Sari MA, Iwasaki M, Shimada T, Kaminsky LS, Guengerich FP: Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in Saccharomyces cerevisiae. Biochemistry 29: 11280-11292, 1990
Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, Molowa DT, Vandenbranden M: Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol 38: 207-213, 1990
Gillam EM, Baba T, Kim BR, Ohmori S, Guengerich FP: Expression of modified human cytochrome P450 3A4 in Escherichia coli and purification and reconstitution of the enzyme. Arch Biochem Biophys 305: 123-131, 1993
Kronbach T, Fischer V, Meyer UA: Cyclosporine metabolism in human liver: Identification of a cytochrome P-450III gene family as the major cyclosporine-metabolizing enzyme explains interactions of cyclosporine with other drugs. Clin Pharmacol Ther 43: 630-635, 1988
Lemoine A, Gautier JC, Azoulay D, Kiffel L, Belloc C, Guengerich FP, Maurel P, Beaune P, Leroux JP: Major pathway of imipramine metabolism is catalyzed by cytochromes P-450 1A2 and P-450 3A4 in human liver. Mol Pharmacol 43: 827-832, 1993
Gonzalez FJ, Song BJ, Hardwick JP: Pregnenolone 16 alpha-carbonitrile-inducible P-450 gene family: Gene conversion and differential regulation. Mol Cell Biol 6: 2969-2976, 1986
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP: Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270: 414-423, 1994
Cupp MJ, Tracy TS: Cytochrome P450: New nomenclature and clinical implications (see comments). Am Fam Physician 57: 107-116, 1998
Waxman DJ, Lapenson DP, Aoyama T, Gelboin HV, Gonzalez FJ, Korzekwa K: Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys 290: 160-166, 1991
Yamazaki H, Shimada T: Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys 346: 161-169, 1997
Graves PE, Kaminsky LS, Halpert J: Evidence for functional and structural multiplicity of pregnenolone-16 alpha-carbonitrile-inducible cytochrome P-450 isozymes in rat liver microsomes. Biochemistry 26: 3887-3894, 1987
Baulieu EE: Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23: 963-987, 1998
Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS: Steroid modulation of the chloride ionophore in rat brain: Structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther 246: 803-812, 1988
Gee KW: Steroid modulation of the GABA/benzodiazepine receptorlinked chloride ionophore. Mol Neurobiol 2: 291-317, 1988
Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G: Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites (In process citation). Eur J Pharmacol 375: 225-235, 1999
Wang H, Kawashima H, Strobel HW: cDNA cloning of a novel CYP3A from rat brain. Biochem Biophys Res Commun 221: 157-162, 1996
Wang H, Strobel HW: Regulation of CYP3A9 gene expression by estrogen and catalytic studies using cytochrome P450 3A9 expressed in Escherichia coli. Arch Biochem Biophys 344: 365-372, 1997
Ribeiro V, Lechner MC: Cloning and characterization of a novel CYP-3A1 allelic variant: Analysis of CYP3A1 and CYP3A2 sex-hormonedependent expression reveals that the CYP3A2 gene is regulated by testosterone. Arch Biochem Biophys 293: 147-152, 1992
Strotkamp D, Roos PH, Hanstein WG: A novel CYP3A gene from female rats. Biochim Biophys Acta 1260: 341-344, 1995
Mahnke A, Strotkamp D, Roos PH, Hanstein WG, Chabot GG, Nef P: Expression and inducibility of cytochrome P450 3A9 (CYP3A9) and other members of the CYP3A subfamily in rat liver. Arch Biochem Biophys 337: 62-68, 1997
Rowlands JC, Hakkak R, Ronis MJJ, Strobel HW, Badger TM: Chronic ethanol treatment differentially affects the expression of CYP3A enzymes in the liver of male rats. Toxicologist 48: 409-410, 1999
Gillam EM, Guo Z, Ueng YF, Yamazaki H, Cock I, Reilly PE, Hooper WD, Guengerich FP: Expression of cytochrome P450 3A5 in Escherichia coli: Effects of 5′ modification, purification, spectral characterization, reconstitution conditions, and catalytic activities. Arch Biochem Biophys 317: 374-384, 1995
Smith PK, Krohn RI, Hermonson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC: Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76-85, 1985
Dignam JD, Strobel HW: NADPH-cytochrome P-450 reductase from rat liver: Purification by affinity chromatography and characterization. Biochemistry 16: 1116-1123, 1977
Shimada T, Misono KS, Guengerich FP: Human liver microsomal cytochrome P-450 mephenytoin 4-hydroxylase, a prototype of genetic polymorphism in oxidative drug metabolism. Purification and characterization of two similar forms involved in the reaction. J Biol Chem 261: 909-921, 1986
Swinney DC, Ryan DE, Thomas PE, Levin W: Evidence for concerted kinetic oxidation of progesterone by purified rat hepatic cytochrome P-450g. Biochemistry 27: 5461-5470, 1988
Shiverick KT, Neims AH: Multiplicity of testosterone hydroxylases in a reconstituted hepatic cytochrome P-450 system from uninduced male 135 rats. Drug Metab Dispos 7: 290-295, 1979
Gonzalez FJ, Nebert DW, Hardwick JP, Kasper CB: Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem 260: 7435-7441, 1985
Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE: Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA 78: 4704-4707, 1981
Baulieu EE: Dehydroepiandrosterone (DHEA): A fountain of youth? (editorial). J Clin Endocrinol Metab 81: 3147-3151, 1996
Baulieu EE, Schumacher M, Koenig H, Jung-Testas I, Akwa Y: Progesterone as a neurosteroid: Actions within the nervous system. Cell Mol Neurobiol 16: 143-154, 1996
Mellon SH: Neurosteroids: Biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab 78: 1003-1008, 1994
Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G: Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol 375: 225-235, 1999
Morfin R, Young J, Corpechot C, Egestad B, Sjovall J, Baulieu EE: Neurosteroids: pregnenolone in human sciatic nerves. Proc Natl Acad Sci USA 89: 6790-6793, 1992
Baulieu EE, Schumacher M, Koenig H, Jung-Testas I, Akwa Y: Progesterone as a neurosteroid: Actions within the nervous system. Cell Mol Neurobiol 16: 143-154, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, H., Napoli, K.L. & Strobel, H.W. Cytochrome P450 3A9 catalyzes the metabolism of progesterone and other steroid hormones. Mol Cell Biochem 213, 127–135 (2000). https://doi.org/10.1023/A:1007124417566
Issue Date:
DOI: https://doi.org/10.1023/A:1007124417566