Abstract
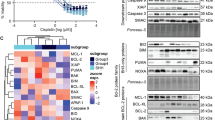
Since apoptosis is the primary mode of cell death induced by cisplatin, the role of apoptosis and apoptosis-related gene products in cisplatin resistance was investigated in four human cisplatin-resistant cell lines of different tumour type. A common feature of the resistant sublines was a reduced susceptibility to drug-induced apoptosis compared to parental sensitive lines. Loss of wild-type p53 function was not a general event associated with the development of drug resistance. An increased bcl-2 expression was found in resistant cells characterized by mutant p53 (A431/Pt and IGROV-1/Pt), whereas in osteosarcoma (U2-OS/Pt) and in ovarian carcinoma (A2780/CP) cells with wild-type p53, bcl-2 levels were markedly reduced. U2-OS/Pt cells had a 16-fold increase in the level of Bcl-xL protein. Stable transfection of U2-OS cells with bcl-xL cDNA conferred a low level of drug resistance to cisplatin, suggesting that overexpression of this gene contributes to the ci splatin-resistant phenotype of this osteosarcoma cell system. In conclusion, these observations suggest a variable contribution of apoptosis-related genes to cisplatin resistance depending on the biological background of the cell system and presumably reflecting different pathways of apoptosis.
Similar content being viewed by others
References
Hickman JA. Apoptosis and chemotherapy resistance. Eur J Cancer 1996; 32A: 921–926.
Nelson WG, Kastan MB. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol 1994; 14: 1815–1823.
Oren M. Relationship of p53 to the control of apoptotic cell death. Cancer Biol 1994; 5: 221–227.
Eliopoulos AG, Kerr DJ, Herod J, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene 1995; 11: 1217–1228.
Milner J. DNA damage, p53 and anticancer therapies. Nat Med 1995; 1: 879–880.
Fan S, El-Deiry WS, Bae I, et al. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA-damaging agents. Cancer Res 1994; 54: 5824–5830.
Tsujimoto Y, Cossman J, Jaffe E, Croce C. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985; 228: 1440.
Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood 1992; 80: 879–886.
Miyashita T, Reed JC. Bcl-2 gene transfer increases relative resistance of 549.1 and WEH17.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res 1992; 52: 5407–5411.
Craig RW. The BCL-2 gene family. Seminars in Cancer Biology 1995; 6: 35–43.
Sato T, Hanada M, Bodrug S, et al. Interactions among members of the Bcl-2 protein family analysed with the yeast two-hybrid system. Proc Natl Acad Sci USA 1994; 91: 9238–9242.
Sedlak TW, Oltvai ZN, Yang E, et al. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA 1995; 92: 7834–7838.
Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodi-merizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993; 74: 609–619.
Boise LH, Gonzales-Garcia M, Postema CE, et al. Bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597–608.
Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Genetics 1993; 90: 3516–3520.
Reynolds JE, Yang T, Quian L, et al. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-myc overexpression in Chinese hamster ovary cells. Cancer Res 1994; 54: 6348–6352.
Farrow SN, White JHM, Martinou I, et al. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature 1995; 374: 731–733.
Chittenden T, Harrington EA, O'Connor R, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature 1995; 374: 733–736.
Kiefer MC, Brauer MJ, Powers VG, et al. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 1995; 374: 736–739.
Perego P, Giarola M, Righetti SC, et al. Association between cisplatin resistance and mutation of p53 and reduced bax expression in ovarian carcinoma cell systems. Cancer Res 1996; 56: 556–562.
Shekan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxic assay for anticancer-drug screening. JNCI 1990; 82: 1107–1112.
Miwa T, Manabe Y, Kurokawa K, et al. Structure, chromosome location, and expression of the human smooth muscle (enteryc type) gamma-actin gene: evolution of six human actin genes. Mol Cell Biol 1991; 11: 3296–3306.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685.
Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed JC. Immunohistochemical analysis of Mcl-1 and Bcl-2 proteins in normal and neoplastic lymph nodes. Am J Pathol 1994; 145: 515–525.
Krajewski S, Blomqvist C, Franssila K, et al. Reduced expression of proapoptotic gene bax is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res 1995; 55: 4471–4478.
Krajewski S, Krajewska M, Shabaik A, et al. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 1994; 54: 5501–5507.
Krajewski S, Bodrug S, Krajewska M, et al. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol 1995; 146: 1309–1318.
Krajewski S, Krajewska M, Reed JC Immunohistochemical analysis of in vivo patterns of bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Res 1996; 56: 2849–2855.
Park DJ, Nakamura H, Chumakov AM, et al. Transactivational and DNA binding abilities of endogenous p53 in p53 mutant cell lines. Oncogene 1994; 9: 1899–1906.
El-Deiry WS, Tokino T, Velculescu VE, et al. WAF, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–825.
Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells 1990; 2: 275–280.
Hickman JA. Apoptosis induced by anticancer drugs. Cancer Met Rev 1992; 11: 121–139.
Silvestrini R, Veneroni S, Daidone MG, et al. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. JNCI 1994; 86: 499–504.
Righetti SC, Della Torre G, Pilotti S, et al. A comparative study of p53 gene mutations, protein accumulation and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res 1996; 56: 689–693.
Reed JC. Bcl-2 family proteins: regulators of chemoresistance in cancer. Toxicol Lett 1995; 82–83: 155–158.
Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of Bcl-xL can confer a multidrug resistance phenotype. Blood 1995; 86: 1903–1910.
Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 1993; 81: 151–157.
Kamesaki S, Kamesaki H, Jorgensen TJ, Tanizawa A, Pommier Y, Cossman J. Bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair. Cancer Res 1993; 53: 4251–4256.
Fisher TC, Milner AE, Gregory CD, et al. Bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is dependent of classical resistance pathways. Cancer Res 1993; 53: 3321–3326.
Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-xL and Bcl-2 repress a common pathway of cell death. J Exp Med 1995; 182: 821–828.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Perego, P., Righetti, S.C., Supino, R. et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis 2, 540–548 (1997). https://doi.org/10.1023/A:1026442716000
Issue Date:
DOI: https://doi.org/10.1023/A:1026442716000