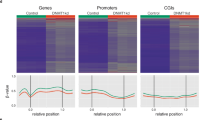
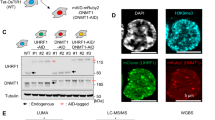
Abstract
Inactivation of tumour suppressor genes is central to the development of all common forms of human cancer1. This inactivation often results from epigenetic silencing associated with hypermethylation rather than intragenic mutations2,3,4,5,6,7. In human cells, the mechanisms underlying locus-specific or global methylation patterns remain unclear8,9. The prototypic DNA methyltransferase, Dnmt1, accounts for most methylation in mouse cells10,11, but human cancer cells lacking DNMT1 retain significant genomic methylation and associated gene silencing12. We disrupted the human DNMT3b gene in a colorectal cancer cell line. This deletion reduced global DNA methylation by less than 3%. Surprisingly, however, genetic disruption of both DNMT1 and DNMT3b nearly eliminated methyltransferase activity, and reduced genomic DNA methylation by greater than 95%. These marked changes resulted in demethylation of repeated sequences, loss of insulin-like growth factor II (IGF2) imprinting, abrogation of silencing of the tumour suppressor gene p16INK4a, and growth suppression. Here we demonstrate that two enzymes cooperatively maintain DNA methylation and gene silencing in human cancer cells, and provide compelling evidence that such methylation is essential for optimal neoplastic proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vogelstein, B. & Kinzler, K. W. The Genetic Basis of Human Cancer (McGraw-Hill Health Professions Division, New York, 1998).
Siegfried, Z. & Cedar, H. DNA methylation: a molecular lock. Curr. Biol. 7, R305–R307 (1997).
Bird, A. P. & Wolffe, A. P. Methylation-induced repression—belts, braces, and chromatin. Cell 99, 451–454 (1999).
Robertson, K. D. & Jones, P. A. DNA methylation: past, present and future directions. Carcinogenesis 21, 461–467 (2000).
Tycko, B. Epigenetic gene silencing in cancer. J. Clin. Invest. 105, 401–407 (2000).
Baylin, S. B. & Herman, J. G. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 16, 168–174 (2000).
Ponder, B. A. Cancer genetics. Nature 411, 336–341 (2001).
Eads, C. A. et al. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 59, 2302–2306 (1999).
Schmutte, C., Yang, A. S., Nguyen, T. T., Beart, R. W. & Jones, P. A. Mechanisms for the involvement of DNA methylation in colon carcinogenesis. Cancer Res. 56, 2375–2381 (1996).
Li, E., Bestor, T. H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Rhee, I. et al. CpG methylation is maintained in human cancer cells lacking DNMT1. Nature 404, 1003–1007 (2000).
Bestor, T. H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 9, 2395–2402 (2000).
Kuo, K. C., McCune, R. A., Gehrke, C. W., Midgett, R. & Ehrlich, M. Quantitative reversed-phase high performance liquid chromatographic determination of major and modified deoxyribonucleosides in DNA. Nucleic Acids Res. 8, 4763–4776 (1980).
Feinberg, A. P., Gehrke, C. W., Kuo, K. C. & Ehrlich, M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 48, 1159–1161 (1988).
Bachman, K. E. et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 59, 798–802 (1999).
Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D. & Baylin, S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA 93, 9821–9826 (1996).
Rainier, S. et al. Relaxation of imprinted genes in human cancer. Nature 362, 747–749 (1993).
Ogawa, O. et al. Constitutional relaxation of insulin-like growth factor II gene imprinting associated with Wilms’ tumour and gigantism. Nature Genet. 5, 408–412 (1993).
Steenman, M. et al. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms’ tumour. Nature Genet. 7, 433–439 (1994).
Cui, H., Horon, I. L., Ohlsson, R., Hamilton, S. R. & Feinberg, A. P. Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nature Med. 4, 1276–1280. (1998).
Uejima, H., Lee, M. P., Cui, H. & Feinberg, A. P. Hot-stop PCR: a simple and general assay for linear quantitation of allele ratios. Nature Genet. 25, 375–376 (2000).
Myohanen, S. K., Baylin, S. B. & Herman, J. G. Hypermethylation can selectively silence individual p16ink4A alleles in neoplasia. Cancer Res. 58, 591–593 (1998).
Okano, M., Xie, S. & Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nature Genet. 19, 219–220 (1998).
Lyko, F. et al. Mammalian (cytosine-5) methyltransferases cause genomic DNA methylation and lethality in Drosophila. Nature Genet. 23, 363–366 (1999).
Santi, D. V., Garrett, C. E. & Barr, P. J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 33, 9–10 (1983).
Juttermann, R., Li, E. & Jaenisch, R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl Acad. Sci. USA 91, 11797–11801 (1994).
Jackson-Grusby, L. et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genet. 27, 31–39 (2001).
Chan, T. A., Hermeking, H., Lengauer, C., Kinzler, K. W. & Vogelstein, B. 14-3-3σ is required to prevent mitotic catastrophe after DNA damage. Nature 401, 616–620 (1999).
Vertino, P. M., Yen, R. W., Gao, J. & Baylin, S. B. De novo methylation of CpG island sequences in human fibroblasts overexpressing DNA (cytosine-5-)-methyltransferase. Mol. Cell Biol. 16, 4555–4565 (1996).
Acknowledgements
We thank S. R. Lee and S. G. Rhee for assistance with the HPLC analysis. This work was supported by the Clayton Fund, the V Foundation, and by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.B.B. is consultant to Tibotec-Virco. Under licensing agreement between the Johns Hopkins University and Tibotec-Virco, M.S.P. was licensed to Tibotec-Virco and S.B.B. is entitled to a share of the royalties received by the University from sales of the licensed technology. The terms of these arrangements are being managed by the University in accordance with its conflict of interest policies.
Supplementary information
Rights and permissions
About this article
Cite this article
Rhee, I., Bachman, K., Park, B. et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416, 552–556 (2002). https://doi.org/10.1038/416552a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416552a
This article is cited by
-
Pervasive promoter hypermethylation of silenced TERT alleles in human cancers
Cellular Oncology (2020)
-
Tumor-suppressive function and mechanism of HOXB13 in right-sided colon cancer
Signal Transduction and Targeted Therapy (2019)
-
Genome-wide Analysis Reveals DNA Methylation Alterations in Obesity Associated with High Risk of Colorectal Cancer
Scientific Reports (2019)
-
Epigenetics of lung cancer: a translational perspective
Cellular Oncology (2019)
-
Long noncoding RNA CASC9.5 promotes the proliferation and metastasis of lung adenocarcinoma
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.