Abstract
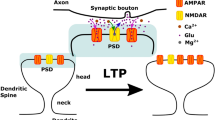
Dendritic spines at excitatory synapses undergo rapid, actin-dependent shape changes which may contribute to plasticity in brain circuits. Here we show that actin dynamics in spines are potently inhibited by activation of either AMPA or NMDA subtype glutamate receptors. Activation of either receptor type inhibited actin-based protrusive activity from the spine head. This blockade of motility caused spines to round up so that spine morphology became both more stable and more regular. Inhibition of spine motility by AMPA receptors was dependent on postsynaptic membrane depolarization and influx of Ca2+ through voltage-activated channels. In combination with previous studies, our results suggest a two-step process in which spines initially formed in response to NMDA receptor activation are subsequently stabilized by AMPA receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Feldman, D. E. & Knudsen, E. I. Experience-dependent plasticity and the maturation of glutamatergic synapses. Neuron 20, 1067–1071 (1998).
Gray, E. G. Electron microscopy of synaptic contacts on dendritic spines of the cerebral cortex. Nature 183, 1592– 1593 (1959).
Carlin, R. K. & Siekevitz, P. Plasticity in the central nervous system: do synapses divide? Proc. Natl. Acad. Sci. USA 80, 3517–3521 (1983).
Harris, K. M. & Kater, S. B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 17, 341–371 (1994).
Frotscher, M., Mannsfeld, B. & Wenzel, J. Environmentally affected differentiation of dendrite spines of pyramidal neurons in rat hippocampus (CA1). J. Hirnforsch 16, 443–450 (1975).
Moser, M. B., Trommald, M. & Andersen, P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. USA 91, 12673–12675 (1994).
Muller, D. Ultrastructural plasticity of excitatory synapses. Rev. Neurosci. 8, 77–93 (1997 ).
Dailey, M. E. & Smith, S. J. The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994 (1996).
Ziv, N. E. & Smith, S. J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 (1996).
Fischer, M., Kaech, S., Knutti, D. & Matus, A. Rapid actin-based plasticity in dendritic spines. Neuron 20, 847–854 (1998).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C. & Yuste, R. Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 96, 13438–13443 (1999).
Lendvai, B., Stern, E. A., Chen, B. & Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000).
McKinney, R. A., Capogna, M., Durr, R., Gahwiler, B. H. & Thompson, S. M. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat. Neurosci. 2, 44–49 (1999).
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Kaech, S., Fisher, M., Doll, T. & Matus, A. Isoform specificity in the relationship of actin to dendritic spines. J. Neurosci. 17, 9565–9572 (1997).
Marin-Padilla, M. Number and distribution of apical dendritic spines of the layer V pyramidal cells in man. J. Comp. Neurol. 131, 475– 490 (1967).
Spacek, J. & Hartmann, M. Three-dimensional analysis of dendritic spines. I. Quantitative observations related to dendritic spine and synaptic morphology in cerebral and cerebellar cortices. Anat. Embryol. (Berl.) 167, 289–310 (1983).
Choi, D. W. Glutamate receptors and the induction of excitotoxic neuronal death. Prog. Brain. Res. 100, 47–51 (1994).
Halpain, S., Hipolito, A. & Saffer, L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844 (1998).
Mayer, M. L., Westbrook, G. L. & Guthrie, P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309, 261–263 (1984).
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A. & Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462–465 (1984).
Allison, D. W., Gelfand, V. I., Spector, I. & Craig, A. M. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J. Neurosci. 18, 2423–2436 (1998).
Guthrie, P. B., Segal, M. & Kater, S. B. Independent regulation of calcium revealed by imaging dendritic spines. Nature 354, 76– 80 (1991).
Denk, W., Sugimori, M. & Llinas, R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc. Natl. Acad. Sci. USA 92, 8279–8282 (1995).
Koester, H. J. & Sakmann, B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc. Natl. Acad. Sci. USA 95, 9596–9601 (1998).
Furukawa, K. et al. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J. Neurosci. 17, 8178– 8186 (1997).
Matus, A. Postsynaptic actin and neuronal plasticity. Curr. Opin. Neurobiol. 9, 561–565 (1999).
Hollmann, M., Hartley, M. & Heinemann, S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252, 851–853 (1991).
Geiger, J. R. et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193– 204 (1995).
Markram, H. & Sakmann, B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc. Natl. Acad. Sci. USA 91, 5207–5211 (1994).
Avery, R. B. & Johnston, D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J. Neurosci. 16, 5567– 5582 (1996).
McDonough, S. I. & Bean, B. P. Mibefradil inhibition of T-type calcium channels in cerebellar purkinje neurons. Mol. Pharmacol. 54, 1080–1087 (1998).
Bartlett, W. P. & Banker, G. A. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. II. Synaptic relationships. J. Neurosci. 4, 1954–1965 (1984).
Boyer, C., Schikorski, T. & Stevens, C. F. Comparison of hippocampal dendritic spines in culture and in brain. J. Neurosci. 18, 5294– 5300 (1998).
Gahwiler, B. H. Development of the hippocampus in vitro: cell types, synapses and receptors. Neuroscience 11, 751– 760 (1984).
Magee, J., Hoffman, D., Colbert, C. & Johnston, D. Electrical and calcium signaling in dendrites of hippocampal pyramidal neurons. Annu. Rev. Physiol. 60, 327–346 (1998).
Svoboda, K. & Mainen, Z. F. Synaptic [Ca2+]: intracellular stores spill their guts. Neuron 22, 427–430 (1999).
Liao, D., Zhang, X., O'Brien, R., Ehlers, M. D. & Huganir, R. L. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat. Neurosci. 2, 37–43 (1999 ).
Petralia, R. S. et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 2, 31–36 (1999 ).
Shi, S. H. et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284 , 1811–1816 (1999).
Kater, S. B. & Mills, L. R. Regulation of growth cone behavior by calcium. J. Neurosci. 11, 891– 899 (1991).
Hong, K., Nishiyama, M., Henley, J., Tessier-Lavigne, M. & Poo, M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403, 93 –98 (2000).
Zheng, J. Q. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403, 89– 93 (2000).
Banker, G. & Goslin, K. in Culturing Nerve Cells (eds. Banker, G. & Goslin, K.) 251–281 (Bradford Books, MIT Press, Cambridge, 1991).
Gahwiler, B. H., Thompson, S. M., Audinat, E. & Robertson, R. T. in Culturing Nerve Cells (eds. Banker, G. & Goslin, K.) 379–411 (Bradford Books, MIT Press, Cambridge, Massachusetts, 1991).
Acknowledgements
We thank Beat Ludin for assistance with image analysis and John Kemp for supplying mibefradil.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figure 1
Glutamate blocks actin dynamics in dendritic spines. This Time Movie-lapse sequence shows GFP-actin dynamics in part of the dendrite shown in Fig. 1. The first section was recorded under control conditions followed by a sequence recorded after 100 µM glutamate had been added to the medium together with 100 µM APV. The high level of actin dynamics seen in these spines under control conditions is typical of all cells maintained in low density culture.
Figure 2
AMPA blocks spine motility in the presence of NMDA receptor antagonists. Effect of AMPA in the presence of MK801. There are two versions of this sequence, Fig2a_1.mov showing the original low magnification recording of a long stretch of dendrite and Fig2a_2.mov showing a small extract of the same sequence at higher magnification. The effects of AMPA both alone and in the presence of the NMDA receptor channel blocker MK801 (10 µM) are documented in this single recording. The first half of this sequence, showing the reversible effect of AMPA on a long stretch of dendrite, was used to prepare the difference images shown in Fig. 5. The sequence shows: 1. (no label) the control condition, when spines are motile; 2. (labeled "+AMPA") a brief period in which the medium was switched to one containing 2 µM AMPA, when spine motility was blocked. Note the rounding up of the spine heads; 3. (no label) an intermediate period when the AMPA was washed out to demonstrate that spine motility recovers; 4. (labeled "+MK801") after the AMPA receptor antagonist MK801 was added to the medium, when spine motility continued unaffected; 5. (labeled +AMPA + MK801) after AMPA was added to the medium in addition to the MK801. During this long final period spine motility was inhibited, showing that it is produced by AMPA receptor stimulation independently of the blocked NMDA receptors.
Figure 2b: Effect of AMPA in the presence of APV. This sequence focuses on part of a dendrite bearing spines with a variety of characteristic morphologies including long necked, mushroom shaped and stubby. The two extracts are from a single long experiment, the first recorded under control conditions, the second in the presence of 1 µM AMPA. 100 µM APV was present throughout the recording to block NMDA receptors. Spines of all categories show actin-based motility under control conditions which is blocked by AMPA. Note that, as in the example shown in Fig2a_2.mov, the effect of AMPA receptor activation is to inhibit the formation of actin-rich protrusions from the spine head.
Figure 3
AMPA blocks spine actin dynamics in organotypic slice cultures. Three video sequences, taken from a time-lapse recording of the 4 week-old slice culture shown in the still frames in the top row of Fig. 3. The sequences are labeled as follows: Fig3_1.mov, spine dynamics in the control condition; Fig3_2.mov recorded in the presence of 1µM AMPA; Fig3_3.mov after washout of the AMPA. Each sequence represents 15 min of recording comprising 60 frames taken at 15 sec intervals. Note the lack of spine dynamics during the sequence shown in Fig3_2, indicating that spine movement seen under control conditions is due to actin dynamics and not focus shift or other artifacts of the recording technique.
Figure 4
Blockade of spine motility by NMDA requires lowering Mg2+ in the medium. This recording begins with a period of control recording followed by a sequence in which 1 µM NMDA was added in regular medium containing 0.5 mM Mg2+ (labeled "+NMDA") which did not inhibit spine motility. The medium was then switched to one containing NMDA and 0.1 mM Mg2+ (labeled +NMDA + low Mg2+) when spine motility was inhibited.
Figure 6
AMPA receptor blockade of spine motility is Na+ dependent. A single time-lapse recording during which the following conditions were applied: 1. (labeled "Control") in control medium the spines on this segment of dendrite were motile; 2. (labeled "-Na") when NaCl was replaced by choline chloride spine motility continued undiminished; 3. (labeled "-Na, +AMPA") subsequently 200nM AMPA was added to the medium but still in the absence of sodium. Despite the presence of AMPA, motility was not noticeably inhibited when Na+ was absent; 4. (labeled "+Na+AMPA") When NaCl was added back to the medium AMPA now blocked spine motility.
Additional data. Blockade of voltage dependent Na+ channels does not affect AMPA-induced inhibition of spine motility. The first part of this recording shows that spine motility remains intact in a cell that had been exposed to 1µM TTX for 2 hours. The second part of the recording shows that adding AMPA (2 µM) still inhibited spine motility even though TTX was present. This particular cell had a well-developed set of recurrent axons which are visible among its dendrites as thinner processes containing faint patches of actin. These are seen to travel along the axons during the recording and this transport is not blocked by AMPA treatment.
Figure 7
Raising [K+]o induces transitory blockade of spine motility. At the time indicated by the appearance of the label "KCl" the external K+ concentration was raised to 8 mM. This produced a transitory inhibition of spine motility and rounding up of spine heads, consistent with the spontaneous recovery of the membrane potential from the temporary depolarization induced by raising the external K+ concentration.
Supplementary data_cd. Voltage-activated calcium channel (VAC) antagonists and AMPA-induced inhibition of spine motility. Fig7_Ni: These 4 excerpts are taken from a single continuous time-lapse recording under the following conditions: 1) a period of control recording in regular Tyrode's solution to establish the base level of motility. 2) After the addition of 100 µM Ni2+, which does not influence spine motility. 3) With the addition of 2 µM AMPA in the continued presence of 10 µM Ni2+. The usual inhibition of motility by AMPA is suppressed by the Ni2+. 4) After changing the medium to one still containing AMPA but now lacking Ni2+. Spine motility is now shows AMPA inhibition. APV (100 µM) to block NMDA receptors was present in all solutions.
Supplementary data_ni. The equivalent experiment to the one described above showing that AMPA can inhibit spine motility in the presence of 500 µM Cd2+.
Supplementary data_nif. Showing that the HVAC antagonist nifedipine (20 µM) does not suppress AMPA inhibition of spine motility.
Rights and permissions
About this article
Cite this article
Fischer, M., Kaech, S., Wagner, U. et al. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci 3, 887–894 (2000). https://doi.org/10.1038/78791
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/78791
This article is cited by
-
Nck1 activity in lateral amygdala regulates long-term fear memory formation
Translational Psychiatry (2022)
-
Functional impact of HIV-1 Tat on cells of the CNS and its role in HAND
Cellular and Molecular Life Sciences (2020)
-
Adolescent social instability stress alters markers of synaptic plasticity and dendritic structure in the medial amygdala and lateral septum in male rats
Brain Structure and Function (2019)
-
Effects of Hippocampal LIMK Inhibition on Memory Acquisition, Consolidation, Retrieval, Reconsolidation, and Extinction
Molecular Neurobiology (2018)
-
The impact of cytoskeletal organization on the local regulation of neuronal transport
Nature Reviews Neuroscience (2017)