Abstract
To date, research on the human ether-a-go-go related gene (hERG) has focused on this potassium channel's role in cardiac repolarization and Long QT Syndrome (LQTS). However, growing evidence implicates hERG in a diversity of physiologic and pathological processes. Here we discuss these other functions of hERG, particularly their impact on diseases beyond cardiac arrhythmia.
Similar content being viewed by others
Introduction
The human ether-a-go-go related gene (hERG) encoded potassium channel has generated considerable scientific interest due to its role in genetically and pharmacologically linked arrhythmias1,2. Admittedly, promiscuous block of cardiac hERG channels by a variety of structurally different drugs represents a major research question and a therapeutic challenge, which has profound impacts on human health. However, its initial discovery was prompted not by cardiac phenomena but by a neurologic phenotype in Drosophila, in which mutation of the homologous Eag gene leads to spasmodic leg movements3,4. Judging by the number of PubMed articles obtained by a search for 'hERG' and 'heart' (627) in comparison to 'cancer' (107), 'brain' (92), or 'pancreas' (4), function of the channel in the nervous system is but one of many topics less prevalent than Long QT Syndrome (LQTS) research. In this perspective we survey existing evidence for hERG expression and function in the other tissues, many of which are linked to disease. Whether its roles are causal or not, these suggest therapeutic opportunities beyond the cardiac system.
Surveying hERG gene expression
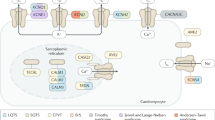
To examine primary evidence for hERG expression in non-cardiac tissues, we utilized NCBI Unigene EST profiles5. Previous analyses have suggested that this type of dataset contains fewer false negatives than microarrays6,7, an appealing characteristic for a broad survey. The results are displayed in Figure 1A, which compares hERG expression to that of three other potassium channels, KCNQ1, Kir2.1 (KCNJ2) (both also expressed in the heart and genetically linked to LQTS) and hEAG (an EAG family member also expressed in cancers). Compared to Kir2.1 and hEAG, hERG is twice and four times, respectively, more broadly expressed across tissues, tumors, and developmental stages. Importantly, KCNQ1 also exhibits similar levels of expression to hERG in these three EST profile sets. We also caution that these data may represent a conservative estimate, as some examples of negative expression in the hERG EST profile, such as breast tumors, contradict existing functional evidence in these cells8,9.
Diversity of tissue expression and regulation of cardiac potassium channels. A) The fraction of samples expressing (gene present/absent) hERG (KCNH2), KCNQ1, Kir2.1 (KCNJ2), or hEAG (KCNH1) channels in Unigene EST profiles for tissues, tumors, and developmental stages. Parentheses indicate the number of samples in each profile class. B) The number of EBI Gene Expression Atlas experiments showing differential expression (transcript regulated up/down) of cardiac potassium channels across five experimental classes.
We also explored information concerning differential expression (DE, significant up- or down-regulation), according to microarray and RNA-Seq meta-analyses in the EBI Gene Expression Atlas10. The results in Figure 1B, like the Unigene profiles, indicate a diversity of tissues and diseases in which hERG is differentially expressed. Intriguingly, even though the metric compared (absence/presence versus DE) is different in the Unigene EST and EBI Gene Atlas data, the relationship between the hERG, KCNQ1, Kir2.1, and hEAG profiles remains similar. While hERG and KCNQ1 demonstrate similar levels of DE across all samples types, Kir2.1 and hEAG have fewer observed cases of DE in the same rank order as the EST data. While a more systematic analysis is outside the scope of this article, we speculate that the similarity in patterns between the presence/absence (EST profiles) and DE (Gene Atlas) data might be explained by more broadly expressed genes possessing greater 'opportunity' for modulation in various diseases or physiological processes.
For each of the tissue types annotated for hERG expression by the EST profile, additional existing evidence through expression, functional studies, or pathologic links are summarized in Table 1.
Roles in cancer
In addition to signaling in the mammalian nervous system, growing evidence shows changes in membrane potential occur during cellular differentiation and cell cycle progression45,46. Thus, it is perhaps unsurprising that changes in the expression of voltage-sensitive channels such as hERG have been reported in cancer, a disease associated with disregulated cellular proliferation. The initial study implicating hERG in oncogenesis utilized both Northern blot probes and patch clamps to identify functional expression of the channel in 17 tumor types derived from diverse cell lineages13. Because corresponding non-pathological tissues for these tumors lacked expression of hERG, the authors proposed that the depolarization resulting from channel over-expression might confer a selective advantage for survival in hypoxic environments13. Additional functional evidence for this interpretation is that Imatinib (a known channel blocker) decreases VEGF secretion in leukemic cells expressing hERG, which could inhibit the growth of endothelial vasculature that supports tumor viability21. Additional experiments studying pharmacological inhibition by E4031 (a type III antiarrhythmic and selective channel blocker) have suggested that hERG expression may facilitate cell migration in diverse hematopoetic neoplasms through an integrin-associated signaling pathway17,19,23. Further, hERG has also been identified in microvesicles shed by leukemic cells22. These microvesicles up-regulate hERG expression in non-neoplastic cells when incorporated in the cell membrane, a feedback mechanism that thus exerts pleiotropic effects through vesicular trafficking22.
In some cancer cell lines, the pharmacological cross-reactivity of hERG and other targets complicates interpretation of its function. This is demonstrated by experiments using MCF-7 breast cancer cells, in which application of the selective inhibitor E4031 has identified a distinct role for hERG in volume regulation that is separate from the proliferation mediated by the closely related human ether-a-go-go gene (hEAG) potassium channel9. These proliferative effects are blocked by astemizole9, which is known to inhibit both hEAG and hERG, while caspase-3 dependent apoptosis may be initiated by the similarly nonspecific effects of arsenic trioxide8. Taken together with previous evidence that associates genetically linked LQTS with mutations in at least eleven genes, including other potassium, calcium, and sodium channels47,48, such data suggest that compounded effects on hERG and other ion-conductive proteins might not be easily separated with nonselective modulators. Indeed, blockade of multiple classes of ion channels may have synergistic effects on tumor growth, as suggested by prostate cancer experiments in which amiodarone (a K+, Ca2+, and Na+ channel blocker) is more potent than compounds that block only two ion channel classes49. Furthermore, natural products such as berberine are thought to have effects not only on multiple ion fluxes, but also on other oncogenic pathways50, thereby complicating the interpretation of their anti-migratory activity in hERG-expressing AML cells23. Additionally, hERG functions in one tissue may be associated with different channels in others. Indeed, in medullablastomas similar volume regulation, as discussed above, has been linked to the EAG2 channel rather than hERG51. Conversely, the specific inhibitors E4031 and WAY have been shown to mediate apoptotic and anti-proliferative effects in leukemia, effects that appear independent of hERG in other tumors52. However, given that the non-selective inhibitor ranolazine (which blocks voltage-gated sodium channels53 as well as hERG54) also inhibits leukemia proliferation55, the effects of blocking multiple ionic currents may be tissue-specific.
The particular cell cycle defects associated with hERG expression may also vary between neoplasms of different tissue origin. Experiments in gastric and ovarian carcinomas suggest that channel function is associated with S-phase transition or accumulation36,41, while in endometrial cancers activity appears to be correlated with occupancy of the G2/M phase43. Cell cycle dependent patterns of channel expression add further complexity to hERG's role in SH-SY5Y neuroblastoma cells30. Furthermore, it remains unclear whether hERG expression in cancerous cells (or nervous system disorders, as discussed below) represents a downstream consequence of general pathologic processes such as inflammation. Evidence for the modulation of hERG expression by inflammation includes down-regulation following ceramide-induced TNF-α signaling56, as well as changes following pro-inflammatory arsenic or mercury treatment57,58. Analogously, data from leukemia suggest that hERG expression may be induced in a dose-dependent manner by chemokine SDF-1a, a constitutively active stromal signaling factor59. As well as being downstream of other signals, hERG expression may conceivably be coordinately regulated with other tumor biomarkers such as the hEAG channel36,51, TNFR127, or CXCR452. Given these mechanisms, the induction of inflammation-associated genes in schizophrenia60 and epilepsy61 suggests the possibility that channel expression might also be induced in neurologic conditions as a secondary consequence of tissue damage in the nervous system.
In contrast to the examples given above, where the absence of the channel in normal tissues suggests its expression might serve as a biomarker for cancer44, the expression of hERG in some tumors may reflect a non-pathogenic role. For example, prolactin secretion in adenomas derived from the pituitary gland is dependent upon hERG expression39. There is also evidence that the channel may not always mediate cancer itself, but rather the physiologic response to the disease. For instance, the murine homologue of hERG is up-regulated in the skeletal muscles of mice whose mobility is reduced due to wasting and inactivity following tumor injection62. This up-regulation subsequently appears to induce muscular atrophy by activating the ubiquitin proteasome system62.
Digestive, secretory, and reproductive systems
Like the heart, the mammalian digestive, secretory, and reproductive systems require electrically coupled contractions. This similarity to cardiac repolarization logically supports a role for hERG in these systems. Indeed, immunohistochemical and pharmacological data argue for the expression of functional hERG channels in both the longitudinal smooth muscles and the enteric neurons of the human small intestine31. These results parallel earlier studies that correlated phasic contractions in the rat stomach with activity of hERG homologues, suggesting that this role was conserved through evolution63. Further, the pH sensitivity of the channel may provide a molecular link for regulating electrical signaling through the acidity of the gastrointestinal lumen64. Channel activity may also explain the cramps and diarrhea caused by antibiotics such as erythromycin, which is a known blocker of hERG65.
Rat ERG channels have also been identified in the kidney, where they display heterogeneous subcellular localization according to nephron segment66. Here, the channel function may be related to volume regulation and osmotic balance during sodium transport67. In the human and mouse pancreas, ERG expression and functional currents have been identified in α and β islet cells37. Pharmacological antagonism of the channel in these cells appears to enhance glucose and arginine-induced insulin secretion and repress glucagon secretion under low glucose conditions by modulating transmembrane calcium fluxes37,38.
In mice, contractions of the uterus in early pregnancy may be enhanced or suppressed by chemical activators or inhibitors of ERG68. However, this activity is lost in later pregnancy, during which other voltage-gated potassium channels of the Kv7 family appear to play a role69. Bovine homologues of hERG appear to also regulate rhythmic contractions in the male reproductive system, as inhibitors such as E4031, haloperidol, and cisapride increase movements of the epididymis that facilitate passage of sperm70. In this context, the channel appears to regulate extracellular calcium influx, as the activity is not sensitive to thapsigargin treatment70. The movement of rat sperm in the epididymal tract is similarly accelerated by the potassium channel blocker sibutramine, although whether this is due to the activity of the rat ERG channel remains unclear71.
Signaling and disease in the nervous system
As previously noted, ERG expression was initially identified in both the mammalian hippocampus and the heart3. Spasmodic motor system signaling that is caused by mutations of the Drosophila ERG channels is reminiscent of the epilepsies that are linked to defects in expression or function of mammalian voltage-gated Kv7 (KCNQ) channels3,72. Although Kv7 channels have been associated with both cardiac arrhythmias and a variety of brain diseases47,72, hERG channels have only recently been associated with diseases of the central nervous system. Expression of a short brain-specific isoform of hERG has been associated with schizophrenia25, while sequence variants may correlate with the efficacy of antipsychotic medications in patients24,73. Analysis identifying the statistically significant co-occurrence of LQTS and epilepsy further implicates the hERG channel in neurologic diseases74.
Evidence for the non-pathologic role of ERG channels in the mammalian nervous system has come from in vitro and in vivo studies in rat and mouse. In mice, functional ERG channels have been identified in brain slices derived from the medial nucleus of the trapezoid body (MNTB) of the auditory brainstem75. Hyperexcitability resulting from E4031 or terfenadine treatment in these slices offers an intriguing mechanism for reports linking LQT events to sudden auditory stimuli76. Functional ERG channels have also been identified in murine mitral/tufted cells of the olfactory bulb, indicating that they may be important in regulating excitability in multiple sensory organs77. In the cerebellum, ERG channels appear to be involved in the control of membrane potential and firing frequency adaptation of Purkinje neurons78. During development, expression in GABAergic neurons of the spinal cord has been implicated in circuit maturation35. Data from rats have also suggested a role for ERG channels in hippocampal γ oscillations, and that they are regulated by thyrotropin-releasing hormone (TRH) signaling in the anterior pituitary gland79,80. In chromaffin cells, ERG activity appears to modulate epinephrine secretion, offering a possible connection between LQTS and catecholaminergic signaling14. In midbrain dopamine neurons, hERG blockers have been shown to limit depolarization inactivation, and thus may have therapeutic benefit for psychiatric diseases associated with defects in dopamine signaling81. Beyond neurons, ERG channels have also been identified in rat microglia82.
Roles in development
In addition to regulating LQTS in adults, hERG, like other potassium channels83, appears to have an important role in development. Data derived from mutational analyses of an Arabian family with frequent miscarriages suggests that homozygous nonsense mutations in the channel may be associated with embryonic lethality15. Functional experiments based on this genetic analysis highlight the nonsense-mediated decay of the hERG transcript and subsequent neonatal arrhythmias as a potential mechanism for this recurrent fetal loss15.
Pharmacologically, hERG-blocking drugs may induce embryonic ischemia by impairing cardiac activity84. This harmful effect is amplified when blood flow is restored due to the generation of reactive oxygen species (ROS), which can lead to developmental abnormalities84, such as cleft palate defects or ventricular malformations observed in rat models28,85. Similar teratogenic effects have been reported for other medications including erythromycin, almokalant, dofetilide, phenytoin, cisapride, and astemizole84,86. Further, it has been demonstrated that progesterone may modulate hERG folding in the ER and Golgi trafficking by regulating intracellular cholesterol homeostasis, thus offering a possible mechanism for arrhythmic risk in late-stage pregnancy87.
Perspective
Although hERG has received attention primarily because of its role in LQTS, our survey highlights the diverse biological and pathogenic roles of the channel. These studies have been catalyzed by the availability of pharmacological agents for hERG channels. This rich functional repertoire has implications for translational research, as potential chemotherapeutic or anti-schizophrenic effects of known blockers must be balanced by consequent concerns for cardiac safety. Indeed, patients who have experienced severe LQT-caused cardiac conditions often also have other complicating life style factors or health conditions. Therefore, LQTS and other medical conditions caused by or linked to hERG cannot readily be separated. In some instances, cardiac side effects may be mitigated by compensatory modulation88,89,90. The promiscuity of drug-channel interactions that is unique to hERG also raises the question of whether there is a much broader but less well characterized impact on health by drugs that are capable of inhibiting hERG currents in non-cardiac cells.
We also note that the majority of activities summarized here are a direct result of a reduction in potassium current densities. However, research also supports the possibility of non-conductive roles for potassium and other ion channels, through signaling that is regulated by proteolytic cleavage of channel proteins91 or activation of classical kinase pathways92. Thus, it is also conceivable that hERG possesses conductance-independent functions that are as-yet not clearly defined. Regardless, the diverse functions of the channel, causal or not, provide evidence that hERG could be targeted in therapies for many non-cardiac diseases, provided that the potential cardiac liabilities can be safely managed.
References
Sanguinetti MC, Jiang CG, Curran ME, Keating MT . A mechanistic link between an inherited and an acquired cardiac arrhythmia: Herg encodes the IKr potassium channel. Cell 1995; 81: 299–307.
Sanguinetti MC, Tristani-Firouzi M . hERG potassium channels and cardiac arrhythmia. Nature 2006; 440: 463–9.
Warmke JW, Ganetzky B . A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A 1994; 91: 3438–42.
Ganetzky B, Wu CF . Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1983; 1: 17–28.
Wheeler DL, Church DM, Federhen S, Lash AE, Madden TL, Pontius JU, et al. Database resources of the national center for biotechnology. Nucleic Acids Res 2003; 31: 28–33.
Zhu J, He F, Song S, Wang J, Yu J . How many human genes can be defined as housekeeping with current expression data? BMC genomics 2008; 9: 172.
Souiai O, Becker E, Prieto C, Benkahla A, De las Rivas J, Brun C . Functional integrative levels in the human interactome recapitulate organ organization. PloS one 2011; 6: e22051.
Wang Y, Zhang Y, Yang L, Cai BZ, Li JP, Zhou Y, et al. Arsenic trioxide induces the apoptosis of human breast cancer MCF-7 cells through activation of caspase-3 and inhibition of HERG channels. Exp Ther Med 2011; 2: 481–6.
Roy J, Vantol B, Cowley EA, Blay J, Linsdell P . Pharmacological separation of hEAG and hERG K+ channel function in the human mammary carcinoma cell line MCF-7. Oncol Rep 2008; 19: 1511–6.
Kapushesky M, Emam I, Holloway E, Kurnosov P, Zorin A, Malone J, et al. Gene expression atlas at the European bioinformatics institute. Nucleic Acids Res 2010; 38: D690–8.
Wymore RS, Gintant GA, Wymore RT, Dixon JE, McKinnon D, Cohen IS . Tissue and species distribution of mRNA for the IKr–like K+ channel, erg. Circ Res 1997; 80: 261–8.
Lim MC, Shipston MJ, Antoni FA . Posttranslational modulation of glucocorticoid feedback inhibition at the pituitary level. Endocrinology 2002; 143: 3796–801.
Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, et al. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer res 1998; 58: 815–22.
Gullo F, Ales E, Rosati B, Lecchi M, Masi A, Guasti L, et al. ERG K+ channel blockade enhances firing and epinephrine secretion in rat chromaffin cells: the missing link to LQT2-related sudden death? FASEB J 2003; 17: 330–2.
Bhuiyan ZA, Momenah TS, Gong Q, Amin AS, Ghamdi SA, Carvalho JS, et al. Recurrent intrauterine fetal loss due to near absence of HERG: clinical and functional characterization of a homozygous nonsense HERG Q1070X mutation. Heart Rhythm 2008; 5: 553–61.
Niemeyer MI, Hougaard C, Hoffmann EK, Jorgensen F, Stutzin A, Sepulveda FV . Characterisation of a cell swelling-activated K+-selective conductance of ehrlich mouse ascites tumour cells. J Physiol 2000; 524: 757–67.
Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, et al. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem 2002; 277: 18528–34.
Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, et al. HERG K+ channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v)beta(3) integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem 2001; 276: 4923–31.
Li H, Liu L, Guo L, Zhang J, Du W, Li X, et al. HERG K+ channel expression in CD34+/CD38–/CD123 (high) cells and primary leukemia cells and analysis of its regulation in leukemia cells. Int J Hematol 2008; 87: 387–92.
Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 2002; 16: 1791–98.
Zheng F, Li H, Liang K, Du Y, Guo D, Huang S . Imatinib has the potential to exert its antileukemia effects by down-regulating hERG1 K+ channels in chronic myelogenous leukemia. Med Oncol 2012; 29: 2127–35.
Zheng F, Li J, Du W, Wang N, Li H, Huang S . Human ether-a-go-go-related gene K+ channels regulate shedding of leukemia cell-derived microvesicles. Leuk Lymphoma 2012; 53: 1592–8.
Li H, Guo L, Jie S, Liu W, Zhu J, Du W, et al. Berberine inhibits SDF-1-induced AML cells and leukemic stem cells migration via regulation of SDF-1 level in bone marrow stromal cells. Biomed Pharmacother 2008; 62 : 573–8.
Volpi S, Heaton C, Mack K, Hamilton JB, Lannan R, Wolfgang CD, et al. Whole genome association study identifies polymorphisms associated with QT prolongation during iloperidone treatment of schizophrenia. Mol Psych 2009; 14: 1024–31.
Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat med 2009; 15: 509–18.
Faravelli L, Arcangeli A, Olivotto M, Wanke E . A HERG-like K+ channel in rat F-11 DRG cell line: pharmacological identification and biophysical characterization. J Physiol 1996; 496: 13–23.
Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res 2002; 62: 4843–8.
Azarbayjani F, Danielsson BR . Embryonic arrhythmia by inhibition of HERG channels: a common hypoxia-related teratogenic mechanism for antiepileptic drugs? Epilepsia 2002; 43: 457–68.
Danielsson BR, Sköld AC, Johansson A, Dillner B, Blomgren B . Teratogenicity by the hERG potassium channel blocking drug almokalant: use of hypoxia marker gives evidence for a hypoxia-related mechanism mediated via embryonic arrhythmia. Toxicol Appl Pharmacol 2003; 193: 168–76.
Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, et al. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem 2003; 278: 2947–55.
Farrelly AM, Ro S, Callaghan BP, Khoyi MA, Fleming N, Horowitz B, et al. Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol Gastrointest Liver Physiol 2003; 284: G883–95.
Wadhwa S, Wadhwa P, Dinda AK, Gupta NP . Differential expression of potassium ion channels in human renal cell carcinoma. Int Urol Nephrol 2009; 41: 251–7.
Waldhauser KM, Brecht K, Hebeisen S, Ha HR, Konrad D, Bur D, et al. Interaction with the hERG channel and cytotoxicity of amiodarone and amiodarone analogues. Br J Pharmacol 2008; 155: 585–95.
Glassmeier G, Hempel K, Wulfsen I, Bauer CK, Schumacher U, Schwarz JR . Inhibition of HERG1 K+ channel protein expression decreases cell proliferation of human small cell lung cancer cells. Pflugers Arch 2012; 463: 365–76.
Furlan F, Taccola G, Grandolfo M, Guasti L, Arcangeli A, Nistri A, et al. ERG conductance expression modulates the excitability of ventral horn GABAergic interneurons that control rhythmic oscillations in the developing mouse spinal cord. J Neurosci 2007; 27: 919–28.
Asher V, Warren A, Shaw R, Sowter H, Bali A, Khan R . The role of Eag and HERG channels in cell proliferation and apoptotic cell death in SK-OV-3 ovarian cancer cell line. Cancer Cell Int 2011; 11: 6.
Hardy AB, Fox JE, Giglou PR, Wijesekara N, Bhattacharjee A, Sultan S, et al. Characterization of Erg K+ channels in alpha- and beta-cells of mouse and human islets. J Biol Chem 2009; 284: 30441–52.
Rosati B, Marchetti P, Crociani O, Lecchi M, Lupi R, Arcangeli A, et al. Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: the role of HERG K(+) channels in firing and release. FASEB J 2000; 14: 2601–10.
Bauer CK, Wulfsen I, Schafer R, Glassmeier G, Wimmers S, Flitsch J, et al. HERG K(+) currents in human prolactin-secreting adenoma cells. Pflugers Arch 2003; 445: 589–600.
Chen BS, Lo YC, Peng H, Hsu TI, Wu SN . Effects of ranolazine, a novel anti-anginal drug, on Ion currents and membrane potential in pituitary tumor GH3 cells and NG108-15 neuronal cells. J Pharmacol Sci 2009; 110: 295–305.
Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM . Expression and significance of HERG protein in gastric cancer. Cancer Biol Ther 2008; 7: 45–50.
London B, Trudeau MC, Newton KP, Beyer AK, Copeland NG, Gilbert DJ, et al. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res 1997; 81: 870–8.
Suzuki T, Takimoto K . Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int J Oncol 2004; 25: 153–9.
Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L, et al. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer 2000; 83: 1722–9.
Blackiston DJ, McLaughlin KA, Levin M . Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 2009; 8: 3519–28.
Wonderlin WF, Strobl JS . Potassium channels, proliferation and G1 progression. J Membr Biol 1996; 154: 91–107.
Roden DM . Long-QT Syndrome. N Engl J Med 2008; 358: 169–76.
Berger SI, Ma'ayan A, Iyengar R . Systems pharmacology of arrhythmias. Sci Signal 2010; 3: ra30.
Abdul M, Hoosein N . Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett 2002; 186: 99–105.
Sun Y, Xun K, Wang Y, Chen X . A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-cancer drugs 2009; 20: 757.
Huang X, Dubuc AM, Hashizume R, Berg J, He Y, Wang J, et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev 2012; 26: 1780–96.
Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, et al. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood 2011; 117: 902–14.
Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, et al. Mechanisms of atrial-selective block of Na(+) channels by ranolazine: I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol 2011; 301: H1606–14.
Rajamani S, Shryock JC, Belardinelli L . Rapid kinetic interactions of ranolazine with HERG K+ current. J Cardiovasc Pharmacol 2008; 51: 581–9.
Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest 2010; 120: 142–56.
Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, et al. Downregulation of the HERG (KCNH2) K(+) channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci 2005; 118: 5325–34.
Mo J, Xia Y, Wade TJ, DeMarini DM, Davidson M, Mumford J . Altered gene expression by low-dose arsenic exposure in humans and cultured cardiomyocytes: assessment by real-time PCR arrays. Int J Environ Res Public Health 2011; 8: 2090–108.
Ayensu WK, Tchounwou PB . Microarray analysis of mercury–induced changes in gene expression in human liver carcinoma (HepG2) cells: importance in immune responses. Int J Environ Res Public Health 2006; 3: 141–73.
Zheng F, Li H, Du W, Huang S . Role of hERG1 K+ channels in leukemia cells as a positive regulator in SDF-1a-induced proliferation. Hematology 2011; 16: 177–84.
Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E . Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 2007; 7: 46.
Vezzani A, French J, Bartfai T, Baram TZ . The role of inflammation in epilepsy. Nat Rev Neurol 2011; 7: 31–40.
Wang X, Hockerman GH, Green HW 3rd, Babbs CF, Mohammad SI, Gerrard D, et al. Merg1a K+ channel induces skeletal muscle atrophy by activating the ubiquitin proteasome pathway. FASEB J 2006; 20: 1531–3.
Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y . Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 2002; 282: G277–87.
Zhou Q, Bett GC . Regulation of the voltage–insensitive step of HERG activation by extracellular pH. Am J Physiol Heart Circ Physiol 2010; 298: H1710–8.
Cvetanovic I, Ranade V, Lin C, Somberg J . The differential antibacterial and gastrointestinal effects of erythromycin and its chiral isolates. Am J Ther 2006; 13: 48–56.
Carrisoza R, Salvador C, Bobadilla NA, Trujillo J, Escobar LI . Expression and immunolocalization of ERG1 potassium channels in the rat kidney. Histochem Cell Biol 2010; 133: 189–99.
Hebert SC, Desir G, Giebisch G, Wang W . Molecular diversity and regulation of renal potassium channels. Physiol Rev 2005; 85: 319–71.
Greenwood IA, Yeung S, Tribe RM, Ohya S . Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol 2009; 587: 2313–26.
McCallum LA, Pierce SL, England SK, Greenwood IA, Tribe RM . The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med 2011; 15: 577–86.
Mewe M, Wulfsen I, Schuster AME, Middendorff R, Glassmeier G, Schwarz JR, et al. Erg K+ channels modulate contractile activity in the bovine epididymal duct. Am J Physiol Regul Integr Comp Physiol 2008; 294: R895–904.
Bellentani FF, Fernandes GSA, Perobelli JE, Pacini ESA, Kiguti LRA, Pupo AS, et al. Acceleration of sperm transit time and reduction of sperm reserves in the epididymis of rats exposed to sibutramine. J Androl 2011; 32: 718.
Jentsch TJ . Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 2000; 1: 21–30.
Apud JA, Zhang F, Decot H, Bigos KL, Weinberger DR . Genetic variation in KCNH2 associated with expression in the brain of a unique hERG isoform modulates treatment response in patients with schizophrenia. Am J Psychiatry 2012; 169: 725–34.
Johnson J, Hofman N, Haglund C, Cascino G, Wilde A, Ackerman M . Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2009; 72: 224–31.
Hardman RM, Forsythe ID . Ether-a-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. J Physiol 2009; 587: 2487–97.
Wilde AAM, Jongbloed RJE, Doevendans PA, Düren DR, Hauer RNW, van Langen IM, et al. Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1). J Am Coll Cardiol 1999; 33: 327–32.
Hirdes W, Napp N, Wulfsen I, Schweizer M, Schwarz JR, Bauer CK . Erg K+ currents modulate excitability in mouse mitral/tufted neurons. Pflugers Arch 2009; 459: 55–70.
Sacco T, Bruno A, Wanke E, Tempia F . Functional roles of an ERG current isolated in cerebellar Purkinje neurons. J Neurophysiol 2003; 90: 1817–28.
Fano S, Çalışkan G, Heinemann U . Differential effects of blockade of ERG channels on gamma oscillations and excitability in rat hippocampal slices. Eur J Neurosci 2012; 36: 3628–35.
Schledermann W, Wulfsen I, Schwarz JR, Bauer CK . Modulation of rat erg1, erg2, erg3, and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. J Physiol 2001; 532: 143–63.
Ji H, Tucker KR, Putzier I, Huertas MA, Horn JP, Canavier CC, et al. Functional characterization of ether-a-go-go-related gene potassium channels in midbrain dopamine neurons-implications for a role in depolarization block. Eur J Neurosci 2012; 36: 2906–16.
Zhou W, Cayabyab FS, Pennefather PS, Schlichter LC, DeCoursey TE . HERG-like K+ channels in microglia. J Gen Physiol 1998; 111: 781–94.
Spitzer NC . Electrical activity in early neuronal development. Nature 2006; 444: 707–12.
Danielsson BR, Danielsson C, Nilsson MF . Embryonic cardiac arrhythmia and generation of reactive oxygen species: common teratogenic mechanism for IKr blocking drugs. Reprod Toxicol 2007; 24: 42–56.
Teng GQ, Zhao X, Lees-Miller JP, Quinn FR, Li P, Rancourt DE, et al. Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality. Circ Res 2008; 103: 1483–91.
Källén BA, Otterblad Olausson P, Danielsson BR . Is erythromycin therapy teratogenic in humans? Reprod Toxicol 2005; 20: 209–14.
Wu ZY, Yu DJ, Soong TW, Dawe GS, Bian JS . Progesterone impairs human ether-a-go-go-related gene (HERG) trafficking by disruption of intracellular cholesterol homeostasis. J Biol Chem 2011; 286: 22186–94.
Zhang S, Zhou Z, Gong Q, Makielski JC, January CT . Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res 1999; 84: 989–98.
Thomas D, Wendt-Nordahl G, Rockl K, Ficker E, Brown AM, Kiehn J . High-affinity blockade of human ether-a-go-go-related gene human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J Pharmacol Exp Ther 2001; 297: 753–61.
Jehle J, Schweizer PA, Katus HA, Thomas D . Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis 2011; 2: e193.
Gomez–Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R . The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell 2006; 127: 591–606.
Hegle AP, Marble DD, Wilson GF . A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc Natl Acad Sci U S A 2006; 103: 2886–91.
Acknowledgements
We thank the colleagues in Min LI's laboratory for valuable discussions, and Alison Neal for editorial assistance. This work is supported by grants to Min LI from the National Institutes of Health (GM078579, MH084691) and Maryland Stem Cell Research Foundation (2010-MSCRFE-0164-00).
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Babcock, J., Li, M. hERG channel function: beyond long QT. Acta Pharmacol Sin 34, 329–335 (2013). https://doi.org/10.1038/aps.2013.6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.6
Keywords
This article is cited by
-
The role of the HERG channel in the secretion of glucagon-like peptide-1 (GLP-1) from murine intestinal L-cells
Current Medicine (2023)
-
Relative positioning of Kv11.1 (hERG) K+ channel cytoplasmic domain-located fluorescent tags toward the plasma membrane
Scientific Reports (2018)
-
Functional characterization of Kv11.1 (hERG) potassium channels split in the voltage-sensing domain
Pflügers Archiv - European Journal of Physiology (2018)
-
Investigation of miscellaneous hERG inhibition in large diverse compound collection using automated patch-clamp assay
Acta Pharmacologica Sinica (2016)
-
hERG1 potassium channel in cancer cells: a tool to reprogram immortality
European Biophysics Journal (2016)