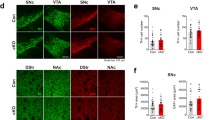
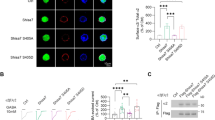
Abstract
Aberrant dopamine D2 receptor (D2R) activity is associated with neuropsychiatric disorders, making those receptors targets for antipsychotic drugs. Here, we report that novel signaling through the intracellularly localized D2R long isoform (D2LR) elicits extracellular signal-regulated kinase (ERK) activation and dendritic spine formation through Rabex-5/platelet-derived growth factor receptor-β (PDGFRβ)-mediated endocytosis in mouse striatum. We found that D2LR directly binds to and activates Rabex-5, promoting early-endosome formation. Endosomes containing D2LR and PDGFRβ are then transported to the Golgi apparatus, where those complexes trigger Gαi3-mediated ERK signaling. Loss of intracellular D2LR-mediated ERK activation decreased neuronal activity and dendritic spine density in striatopallidal medium spiny neurons (MSNs). In addition, dendritic spine density in striatopallidal MSNs significantly increased following treatment of striatal slices from wild-type mice with quinpirole, a D2R agonist, but those changes were lacking in D2LR knockout mice. Moreover, intracellular D2LR signaling mediated effects of a typical antipsychotic drug, haloperidol, in inducing catalepsy behavior. Taken together, intracellular D2LR signaling through Rabex-5/PDGFRβ is critical for ERK activation, dendritic spine formation and neuronal activity in striatopallidal MSNs of mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Missale C, Nash R, Robinson SW, Jaber M, Caron MG . Dopamine receptors from structure to function. Physiol Rev 1998; 78: 189–225.
Seeman P, Lee T, Chau-wong M, Wong K . Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 1976; 261: 717–719.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Tan EK, Khajavi M, Thornby JI, Nagamitsu S, Jankovic J, Ashizawa T . Variability and validity of polymorphism association studies in Parkinson's disease. Neurology 2000; 55: 533–538.
Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A et al. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J 1989; 8: 4025–4034.
Fetsko LA, Xu R, Wang Y . Effects of age and dopamine D2L receptor-deficiency on motor and learning functions. Neurobiol Aging 2005; 26: 521–530.
Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA 2007; 104: 20552–20557.
Beaulieu JM, Gainetdinov RR . The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63: 182–217.
Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG . An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122: 261–273.
Brami-Cherrier K, Valjent E, Garcia M, Pagès C, Hipskind RA, Caboche J . Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 2002; 22: 8911–8921.
Yan Z, Feng J, Fienberg AA, Greengard P . D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci USA 1999; 96: 11607–11612.
Luo Y, Kokkonen GC, Wang X, Neve KA, Roth GS . D2 dopamine receptors stimulate mitogenesis through pertussis toxin-sensitive G proteins and Ras-involved ERK and SAP/JNK pathways in rat C6-D2L glioma cells. J Neurochem 1998; 71: 980–990.
Choi EY, Jeong D, Park KW, Baik JH . G protein mediated mitogen-activated protein kinase activation by two dopamine D2 receptors. Biochem Biophys Res Commun 1999; 256: 33–40.
Takeuchi Y, Fukunaga K . Differential regulation of NF-kappaB, SRE and CRE by dopamine D1 and D2 receptors in transfected NG108-15 cells. J Neurochem 2003; 85: 729–739.
Takeuchi Y, Fukunaga K . Different effects of five dopamine receptor subtypes on nuclear factor-kappaB activity in NG108-15 cells and mouse brain. J Neurochem 2004; 88: 41–50.
Oak JN, Lavine N, Van Tol HH . Dopamine D4 and D2L receptor stimulation of the mitogen-activated protein kinase pathway is dependent on trans-activation of the platelet-derived growth factor receptor. Mol Pharmacol 2001; 60: 92–103.
Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH et al. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron 2002; 35: 1111–1122.
Beazely MA, Tong A, Wei WL, Van Tol H, Sidhu B, MacDonald JF . D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptor-dependent. J Neurochem 2006; 98: 1657–1663.
Horiuchi H, Lippé R, McBride HM, Rubino M, Woodman P, Stenmark H et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 1997; 90: 1149–1159.
Shioda N, Moriguchi S, Oya T, Ishii Y, Shen J, Matsushima T et al. Aberrant hippocampal spine morphology and impaired memory formation in neuronal platelet-derived growth factor β-receptor lacking mice. Hippocampus 2012; 22: 1371–1378.
Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB . Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci 2000; 20: 8305–8314.
Yamaguchi H, Aiba A, Nakamura K, Nakao K, Sakagami H, Goto K et al. Dopamine D2 receptor plays a critical role in cell proliferation and proopiomelanocortin expression in the pituitary. Genes Cells 1996; 1: 253–268.
Mori Y, Matsui T, Fukuda M . Rabex-5 protein regulates dendritic localization of small GTPase Rab17 and neurite morphogenesis in hippocampal neurons. J Biol Chem 2013; 288: 9835–9847.
Wise A, Watson-Koken MA, Rees S, Lee M, Milligan G . Interactions of the α2A-adrenoceptor with multiple Gi-family G-proteins: studies with pertussis toxin-resistant G-protein mutants. Biochem J 1997; 321: 721–728.
Shioda N, Yamamoto Y, Watanabe M, Binas B, Owada Y, Fukunaga K . Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J Neurosci 2010; 30: 3146–3155.
Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA . CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 2011; 7: 624–630.
Liu J, Lamb D, Chou MM, Li YJ, Li G . Nerve growth factor-mediated neurite outgrowth via regulation of Rab5. Mol Biol Cell 2007; 18: 1375–1384.
Manders EM, Verbeek FJ, Aten JA . Measurement of co-localization of objects in dual color confocal images. J Microsc 1993; 169: 375–382.
Shioda N, Beppu H, Fukuda T, Li E, Kitajima I, Fukunaga K . Aberrant calcium/calmodulin-dependent protein kinase II (CaMKII) activity is associated with abnormal dendritic spine morphology in the ATRX mutant mouse brain. J Neurosci 2011; 31: 346–358.
Roy A, Kucukural A, Zhang Y . I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 2010; 5: 725–738.
Lee S, Tsai YC, Mattera R, Smith WJ, Kostelansky MS, Weissman AM et al. Structural basis for ubiquitin recognition and autoubiquitination by Rabex-5. Nat Struct Mol Biol 2006; 13: 264–271.
Penengo L, Mapelli M, Murachelli AG, Confalonieri S, Magri L, Musacchio A et al. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell 2006; 124: 1183–1195.
Delprato A, Merithew E, Lambright DG . Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell 2004; 118: 607–617.
Delprato A, Lambright DG . Structural basis for Rab GTPase activation by VPS9 domain exchange factors. Nat Struct Mol Biol 2007; 14: 406–412.
Takeuchi Y, Fukunaga K . Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells. J Neurochem 2003; 85: 1064–1074.
Bonifacino JS, Rojas R . Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 8: 568–579.
Christoforidis S, McBride HM, Burgoyne RD, Zerial M . The Rab5 effector EEA1 is a core component of endosome docking. Nature 1999; 397: 621–625.
Marchese A, Paing MM, Temple BR, Trejo JG . protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 2008; 48: 601–629.
Curran EJ, Watson SJ Jr . Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. J Comp Neurol 1995; 361: 57–76.
Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M . The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992; 70: 715–728.
Somsel Rodman J, Wandinger-Ness A . Rab GTPases coordinate endocytosis. J Cell Sci 2000; 113: 183–192.
Dou Z, Pan JA, Dbouk HA, Ballou LM, DeLeon JL, Fan Y, Class IA et al. PI3K p110β subunit promotes autophagy through Rab5 small GTPase in response to growth factor limitation. Mol Cell 2013; 50: 29–42.
Le-Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG . Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem 2005; 280: 22012–22020.
Zhai S, Ark ED, Parra-Bueno P, Yasuda R . Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science 2013; 342: 1107–1111.
Shiflett MW, Balleine BW . Contributions of ERK signaling in the striatum to instrumental learning and performance. Behav Brain Res 2011; 218: 240–247.
Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000; 408: 199–203.
Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 2006; 3: 995–1000.
Zhang Z, Zhang T, Wang S, Gong Z, Tang C, Chen J et al. Molecular mechanism for Rabex-5 GEF activation by Rabaptin-5. Elife 2014; 3: e02687.
Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M . Endocytosis promotes rapid dopaminergic signaling. Neuron 2011; 71: 278–290.
Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 2009; 7: e1000172.
Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K . Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 2009; 5: 428–434.
Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB . Assembly and trafficking of heterotrimeric G proteins. Biochemistry 2007; 46: 7665–7677.
Lajiness ME, Chio CL, Huff RM . D2 dopamine receptor stimulation of mitogenesis in transfected Chinese hamster ovary cells: relationship to dopamine stimulation of tyrosine phosphorylations. J Pharmacol Exp Ther 1993; 267: 1573–1581.
van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E et al. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature 1995; 376: 781–784.
Shah BH, Catt KJ . GPCR-mediated transactivation of RTKs in the CNS: mechanisms and consequences. Trends Neurosci 2004; 27: 48–53.
Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol 2005; 170: 607–618.
Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 1998; 394: 494–498.
Waters CM, Connell MC, Pyne S, Pyne NJ . c-Src is involved in regulating signal transmission from PDGFbeta receptor-GPCR(s) complexes in mammalian cells. Cell Signal 2005; 17: 263–277.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 2008; 28: 5671–5685.
Gangarossa G, Perroy J, Valjent E . Combinatorial topography and cell-type specific regulation of the ERK pathway by dopaminergic agonists in the mouse striatum. Brain Struct Funct 2013; 218: 405–419.
Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G et al. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience 1997; 79: 323–327.
Centonze D, Usiello A, Gubellini P, Pisani A, Borrelli E, Bernardi G et al. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology 2002; 27: 723–276.
Dubé L, Smith AD, Bolam JP . Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol 1988; 267: 455–471.
Holt DJ, Graybiel AM, Saper CB . Neurochemical architecture of the human striatum. J Comp Neurol 1997; 384: 1–25.
Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G . Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J Neurosci 2000; 20: RC69.
Fisher RS, Levine MS, Sibley DR, Ariano MA . D2 dopamine receptor protein location: Golgi impregnation-gold toned and ultrastructural analysis of the rat neostriatum. J Neurosci Res 1994; 38: 551–564.
Sesack SR, Aoki C, Pickel VM . Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 1994; 14: 88–106.
Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 2004; 42: 653–663.
Welter M, Vallone D, Samad TA, Meziane H, Usiello A, Borrelli E . Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA 2007; 104: 6840–6845.
Cepeda C, Hurst RS, Altemus KL, Flores-Hernández J, Calvert CR, Jokel ES et al. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol 2001; 85: 659–670.
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 1996; 93: 3679–3683.
Kelleher RJ 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S . Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 2004; 116: 467–479.
Thomas GM, Huganir RL . MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 2004; 5: 173–183.
Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 2006; 9: 251–259.
Villalba RM, Smith Y . Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine? Neuroscience 2013; 251: 2–20.
Acknowledgements
We thank Dr Mitsunori Fukuda (Tohoku University, Sendai, Japan) for kindly providing Rabex-5, Rabex-5-D313A, Rabex-5C, Rabex-5C-D313A and Rabex-5 shRNA plasmids; Dr Wei-Xing Zong (Stony Brook University, Stony Brook, NY, USA) for kindly providing Rab5(WT), Rab5(S34N) and Rab5(Q79L) plasmids; Dr Guangpu Li (University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA) for kindly providing GST-R5BD; and Dr Jonathan A. Javitch (Columbia University, New York, NY, USA) for kindly providing D2LR-RLuc8, D2SR-RLuc8, Gαi1-mVenus, Gαi2-mVenus and Gαi3-mVenus plasmids. This work was supported by Grants-in-Aid for Scientific Research on Innovative Area “Foundation of Synapse and Neurocircuit Pathology” from the Ministry of Education, Culture, Sports, Science and Technology, Japan (25110705 and 25460090 to NS) and (24102505 and 25293124 to KF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Shioda, N., Yabuki, Y., Wang, Y. et al. Endocytosis following dopamine D2 receptor activation is critical for neuronal activity and dendritic spine formation via Rabex-5/PDGFRβ signaling in striatopallidal medium spiny neurons. Mol Psychiatry 22, 1205–1222 (2017). https://doi.org/10.1038/mp.2016.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.200
This article is cited by
-
The Altered Supramolecular Structure of Dopamine D2 Receptors in Disc1-deficient Mice
Scientific Reports (2018)
-
Targeting G-quadruplex DNA as cognitive function therapy for ATR-X syndrome
Nature Medicine (2018)