Abstract
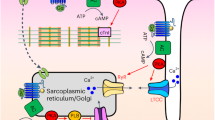
Cyclic adenosine 3′, 5′-monophosphate (cAMP) is a ubiquitous mediator of intracellular signalling events. It acts principally through stimulation of cAMP-dependent protein kinases (PKAs)1,2 but also activates certain ion channels and guanine nucleotide exchange factors (Epacs)3. Metabolism of cAMP is catalysed by phosphodiesterases (PDEs)4,5. Here we identify a cAMP-responsive signalling complex maintained by the muscle-specific A-kinase anchoring protein (mAKAP) that includes PKA, PDE4D3 and Epac1. These intermolecular interactions facilitate the dissemination of distinct cAMP signals through each effector protein. Anchored PKA stimulates PDE4D3 to reduce local cAMP concentrations, whereas an mAKAP-associated ERK5 kinase module suppresses PDE4D3. PDE4D3 also functions as an adaptor protein that recruits Epac1, an exchange factor for the small GTPase Rap1, to enable cAMP-dependent attenuation of ERK5. Pharmacological and molecular manipulations of the mAKAP complex show that anchored ERK5 can induce cardiomyocyte hypertrophy. Thus, two coupled cAMP-dependent feedback loops are coordinated within the context of the mAKAP complex, suggesting that local control of cAMP signalling by AKAP proteins is more intricate than previously appreciated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walsh, D. A., Perkins, J. P. & Krebs, E. G. An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem. 243, 3763–3765 (1968)
Su, Y. et al. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding proteins. Science 269, 807–813 (1995)
Bos, J. L. Epac: a new cAMP target and new avenues in cAMP research. Nature Rev. Mol. Cell Biol. 4, 733–738 (2003)
Beavo, J. A. & Brunton, L. L. Cyclic nucleotide research—still expanding after half a century. Nature Rev. Mol. Cell Biol. 3, 710–718 (2002)
Houslay, M. D. & Adams, D. R. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370, 1–18 (2003)
Perry, S. J. et al. Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science 298, 834–836 (2002)
Verde, I. et al. Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 276, 11189–11198 (2001)
Dodge, K. L. et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signalling module. EMBO J. 20, 1921–1930 (2001)
Carlisle Michel, J. J. et al. PKA phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem. J. 381, 587–592 (2004)
Sette, C. & Conti, M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. J. Biol. Chem. 271, 16526–16534 (1996)
Wong, W. & Scott, J. D. AKAP signalling complexes: Focal points in space and time. Nature Rev. Mol. Cell Biol. 5, 959–971 (2004)
Zhang, J., Ma, Y., Taylor, S. S. & Tsien, R. Y. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl Acad. Sci. USA 98, 14997–15002 (2001)
Hoffmann, R., Baillie, G. S., MacKenzie, S. J., Yarwood, S. J. & Houslay, M. D. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 18, 893–903 (1999)
Zhou, G., Bao, Z. Q. & Dixon, J. E. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 270, 12665–12669 (1995)
English, J. M., Vanderbilt, C. A., Xu, S., Marcus, S. & Cobb, M. H. Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem. 270, 28897–28902 (1995)
Kapiloff, M. S., Schillace, R. V., Westphal, A. M. & Scott, J. D. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J. Cell Sci. 112, 2725–2736 (1999)
MacKenzie, S. J., Baillie, G. S., McPhee, I., Bolger, G. B. & Houslay, M. D. ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases. The involvement of COOH-terminal docking sites and NH2-terminal UCR regions. J. Biol. Chem. 275, 16609–16617 (2000)
Pearson, G. W. & Cobb, M. H. Cell condition-dependent regulation of ERK5 by cAMP. J. Biol. Chem. 277, 48094–48098 (2002)
Cook, S. J. & McCormick, F. Inhibition by cAMP of Ras-dependent activation of Raf. Science 262, 1069–1072 (1993)
Vaillancourt, R. R., Gardner, A. M. & Johnson, G. L. B-Raf-dependent regulation of the MEK-1/mitogen-activated protein kinase pathway in PC12 cells and regulation by cyclic AMP. Mol. Cell. Biol. 14, 6522–6530 (1994)
de Rooij, J. et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 (1998)
Kawasaki, H. et al. A family of cAMP-binding proteins that directly activate Rap1. Science 282, 2275–2279 (1998)
Enserink, J. M. et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nature Cell Biol. 4, 901–906 (2002)
Rubinfeld, B. et al. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell 65, 1033–1042 (1991)
Nicol, R. L. et al. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 20, 2757–2767 (2001)
Zaccolo, M. & Pozzan, T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711–1715 (2002)
Mongillo, M. et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ. Res. 95, 67–75 (2004)
Laroche-Joubert, N., Marsy, S., Michelet, S., Imbert-Teboul, M. & Doucet, A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J. Biol. Chem. 277, 18598–18604 (2002)
Beavo, J. A., Bechtel, P. J. & Krebs, E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 38, 299–308 (1974)
Kodama, H. et al. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 279, H1635–H1644 (2000)
Zhang, J., Hupfeld, C. J., Taylor, S. S., Olefsky, J. M. & Tsien, R. Y. Insulin disrupts β-adrenergic signalling to protein kinase A in adipocytes. Nature doi:10.1038/nature04140 (this issue)
Acknowledgements
This work was supported by grants from the National Institutes of Health (to J.D.S. and M.S.K.) and the American Heart Association (to K.L.D.-K.). The authors wish to thank N. Mayer, D. Bleckinger and R. Mouton for technical assistance, and R. Tsien, M. Houslay, J. E. Dixon, J. L. Bos and P. Stork for reagents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Figure Legends and the Supplementary Video Legends. (DOC 32 kb)
Supplementary Video S1
This representative movie shows HeLa cells transfected with the AKAR-PKA reporter and treated with forskolin. (MOV 996 kb)
Supplementary Video S2
This representative movie shows HeLa cells transfected with the AKAR-PKA-PDE reporter and treated with forskolin. (MOV 817 kb)
Supplementary Video S3
This representative movie shows HeLa cells transfected with the AKAR-PKA-PDE reporter, pre-treated with either milrinone or rolipram, and then treated with forskolin. (MOV 1365 kb)
Supplementary Figures S1 and S2
Supplementary Figure S1: mAKAP and ERK5 co-localize at the nuclear membrane of hypertrophic rat neonatal ventriculocytes (RNV). Supplementary Figure S2: mAKAP scaffolds PDE4D3 and ERK5 to form a ternary complex (PDF 126 kb)
Supplementary Figures S3 and S4
Supplementary Figure S3: mapping of the ERK5 binding fragment on mAKAP. Supplementary Figure S4: PDE4D3 links ERK5 to mAKAP. (PDF 99 kb)
Supplementary Figures S5 and S6
Supplementary Figure S5. mAKAP-anchored PDE activity is negatively regulated by ERK. Supplementary Figure S6. PDE4D3 serves as an adapter protein to tether Epac1 to the mAKAP complex. (PDF 94 kb)
Supplementary Figures S7 and S8
Supplementary Figure S7: PDE4D3 tethers Epac1 to the mAKAP complex. Supplementary Figure S8: PDE4D3 binds directly to both Epac1 and ERK5. (PDF 84 kb)
Supplementary Figures S9 and S10
Supplementary Figure S9: mAKAP silenced RNV exhibit reduced hypertrophy in response to LIF stimulation. Supplementary Figure S10: expression of the mAKAP 585-1286 fragment displaces endogenous mAKAP and ablates LIF induced hypertrophy in RNV. (PDF 140 kb)
Supplementary Figures S11 and S12
Supplementary Figure S11: mAKAP plays a role in LIF-induced cardiac hypertrophy. Supplementary Figure S12: displacement of mAKAP inhibits LIF-induced expression of ANF, an indicator of hypertrophy. (PDF 143 kb)
Rights and permissions
About this article
Cite this article
Dodge-Kafka, K., Soughayer, J., Pare, G. et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 (2005). https://doi.org/10.1038/nature03966
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03966
This article is cited by
-
Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure
Nature Reviews Cardiology (2023)
-
Current and emerging drug targets in heart failure treatment
Heart Failure Reviews (2022)
-
AKAP5 complex facilitates purinergic modulation of vascular L-type Ca2+ channel CaV1.2
Nature Communications (2020)
-
The Popeye domain containing gene family encoding a family of cAMP-effector proteins with important functions in striated muscle and beyond
Journal of Muscle Research and Cell Motility (2019)
-
Leucine-rich repeat kinase 2 controls protein kinase A activation state through phosphodiesterase 4
Journal of Neuroinflammation (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.