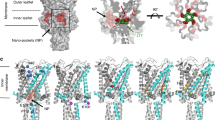
Abstract
Activation of mechanosensitive ion channels by physical force underlies many physiological processes including the sensation of touch, hearing and pain1,2,3,4,5. TRAAK (also known as KCNK4) ion channels are neuronally expressed members of the two-pore domain K+ (K2P) channel family and are mechanosensitive6. They are involved in controlling mechanical and temperature nociception in mice7. Mechanosensitivity of TRAAK is mediated directly through the lipid bilayer—it is a membrane-tension-gated channel8. However, the molecular mechanism of TRAAK channel gating and mechanosensitivity is unknown. Here we present crystal structures of TRAAK in conductive and non-conductive conformations defined by the presence of permeant ions along the conduction pathway. In the non-conductive state, a lipid acyl chain accesses the channel cavity through a 5 Å-wide lateral opening in the membrane inner leaflet and physically blocks ion passage. In the conductive state, rotation of a transmembrane helix (TM4) about a central hinge seals the intramembrane opening, preventing lipid block of the cavity and permitting ion entry. Additional rotation of a membrane interacting TM2–TM3 segment, unique to mechanosensitive K2Ps, against TM4 may further stabilize the conductive conformation. Comparison of the structures reveals a biophysical explanation for TRAAK mechanosensitivity—an expansion in cross-sectional area up to 2.7 nm2 in the conductive state is expected to create a membrane-tension-dependent energy difference between conformations that promotes force activation. Our results show how tension of the lipid bilayer can be harnessed to control gating and mechanosensitivity of a eukaryotic ion channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arnadóttir, J. & Chalfie, M. Eukaryotic mechanosensitive channels. Ann. Rev. Biophysics 39, 111–137 (2010)
Bautista, D. M. & Lumpkin, E. A. Probing mammalian touch transduction. J. Gen. Physiol. 138, 291–301 (2011)
Delmas, P. & Coste, B. Mechano-gated ion channels in sensory systems. Cell 155, 278–284 (2013)
Nilius, B. & Honore, E. Sensing pressure with ion channels. Trends Neurosci. 35, 477–486 (2012)
Anishkin, A., Loukin, S. H., Teng, J. & Kung, C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl Acad. Sci. USA 111, 7898–7905 (2014)
Fink, M. et al. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 17, 3297–3308 (1998)
Noël, J. et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318 (2009)
Brohawn, S. G., Su, Z. & MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl Acad. Sci. USA 111, 3614–3619 (2014)
Kung, C., Martinac, B. & Sukharev, S. I. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313–329 (2010)
Sukharev, S. I., Blount, P., Martinac, B. & Kung, C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59, 633–657 (1997)
Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A. & Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 (2002)
Brohawn, S. G., del Mármol, J. & MacKinnon, R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 (2012)
Brohawn, S. G., Campbell, E. B. & MacKinnon, R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc. Natl Acad. Sci. USA 110, 2129–2134 (2013)
Miller, A. N. & Long, S. B. Crystal structure of the human two–pore domain potassium channel K2P1. Science 335, 432–436 (2012)
del Camino, D. & Yellen, G. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron 32, 649–656 (2001)
Jiang, Y. et al. The open pore conformation of potassium channels. Nature 417, 523–526 (2002)
Harinath, S. & Sikdar, S. K. Trichloroethanol enhances the activity of recombinant human TREK-1 and TRAAK channels. Neuropharmacology 46, 750–760 (2004)
Phillips, R., Ursell, T., Wiggins, P. & Sens, P. Emerging roles for lipids in shaping membrane-protein function. Nature 459, 379–385 (2009)
Opsahl, L. R. & Webb, W. W. Lipid–glass adhesion in giga-sealed patch-clamped membranes. Biophys. J. 66, 75–79 (1994)
Ursell, T., Kondev, J., Reeves, D., Wiggins, P. A. & Phillips, R. in Mechanosensitive Ion Channels (eds Kamkin, A. & Kiseleva, I. ) 1, 37–70 (Springer, 2008)
Enyedi, P. & Czirják, G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605 (2010)
Honoré, E., Patel, A. J., Chemin, J., Suchyna, T. & Sachs, F. Desensitization of mechano-gated K2P channels. Proc. Natl Acad. Sci. USA 103, 6859–6864 (2006)
Maksaev, G., Milac, A., Anishkin, A., Guy, H. R. & Sukharev, S. I. Analyses of gating thermodynamics and effects of deletions in the mechanosensitive channel TREK-1: comparisons with structural models. Channels 5, 26–34 (2011)
Chemin, J. et al. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 24, 44–53 (2005)
Murbartián, J., Lei, Q., Sando, J. J. & Bayliss, D. A. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J. Biol. Chem. 280, 30175–30184 (2005)
Patel, A. J. et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 (1998)
Honoré, E., Maingret, F., Lazdunski, M. & Patel, A. J. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 21, 2968–2976 (2002)
Bagriantsev, S. N., Clark, K. A. & Minor, D. L. Metabolic and thermal stimuli control K2P2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 31, 3297–3308 (2012)
Aryal, P., Abd-Wahab, F., Bucci, G., Sansom, M. S. P. & Tucker, S. J. A hydrophobic barrier deep within the inner pore of the TWIK-1 K2P potassium channel. Nature Commun. 5, 4377 (2014)
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution–from diffraction images to an initial model in minutes. Acta Crystallogr. D 62, 859–866 (2006)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Karplus, P. A. & Diederichs, K. Linking crystallographic model and data quality. Science 336, 1030–1033 (2012)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Cryst. D66, 12–21 (2010)
Read, R. J. & McCoy, A. J. Using SAD data in Phaser. Acta Crystallogr. D 67, 338–344 (2011)
Brooks, B. R. et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009)
Jo, S., Kim, T. & Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2, e880 (2007)
Smart, O. S., Goodfellow, J. M. & Wallace, B. A. The pore dimensions of gramicidin A. Biophys. J. 65, 2455–2460 (1993)
Pettersen, E. F., Goddard, T. D. & Huang, C. C. UCSF Chimera—a visualization system for exploratory research and analysis. J. Computational Chem. 25, 1605–1612 (2004)
Acknowledgements
We thank staff at APS beamlines 23-IDB/D, especially R. Sanishvili and S. Corcoran and at 24-IDC/E, especially I. Kourinov, D. Neau and K. Rajashankar for assistance at the synchrotron and members of the MacKinnon laboratory for discussions. S.G.B. is a Howard Hughes Medical Institute postdoctoral fellow of the Helen Hay Whitney Foundation and R.M. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
S.G.B designed and performed the experiments. E.B.C. performed hybridoma cell culture. R.M. supervised the project. S.G.B and R.M. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 The central cavity in conductive and non-conductive TRAAK conformations.
a, b, View from the membrane plane of the TRAAK central cavity in the non-conductive (a) and conductive (b) conformations. The exposed surface of hydrophobic amino acids are colored white, arginine and lysine are blue, glutamate and aspartate are red, and polar residues are green. The positions of K+ ions in the filter are outlined and residue T277 in TM4 is indicated with an asterisk. c, Diameter of the ion conduction pathway as a function of distance through the membrane for non-conductive TRAAK (red), conductive TRAAK (blue) and TWIK1 (grey, PDB: 3UKM). The green box indicates the position of the selectivity filter and dashed grey lines are the approximate boundaries of the lipid membrane. The ∼10 Å diameter constriction formed partially by T277 is indicated with an asterisk. The pore diameter is larger in TRAAK than in TWIK1 and expands below T277 in the conductive conformation.
Extended Data Figure 2 Reconstituted TRAAK activity in different lipids.
a, Current recorded from TRAAK proteoliposome patches as a function of holding voltage (mean ± s.e.m., n = 9 patches each from three separate experiments). Current through TRAAK reconstituted in phosphatidylcholine lipids with branched acyl chains (1,2-diphytanoyl-sn-glycero-3-phosphocholine, DPhPC) was significantly higher than in non-branched acyl chains (egg l-α-phosphatidylcholine, PC) at each voltage measured (5.0-fold higher at 0 mV, P < 0.0001, Student’s t-test). b, c, Representative recording of pressure (lower trace) activation of TRAAK current (upper trace) in PC (b) or DPhPC (c) lipids. d, Quantification of pressure activation of TRAAK in PC and DPhPC (mean fold pressure activation at 0 mV ± s.e.m., n = 9 patches each from three separate experiments, ***P < 0.0001, Student’s t-test).
Extended Data Figure 3 Representative electrophysiological recordings from wild-type TRAAK and TRAAK(I159C, R284C).
In these experiments and those in Fig. 2, inside-out patches from cells expressing wild-type or mutant TRAAK channels were excised and perfused with reducing bath solution (with 10 mM DTT). After stabilization of the patch (TRAAK channels exhibit a gradual run-up of current following excision to an equilibrium value, for example, Extended Data Fig. 4), the perfusion solution was switched to oxidizing bath solution (no DTT). a, b, Representative voltage family from a TRAAK(I159C, R284C) patch during perfusion of reducing (a) and oxidizing (b) solution. The voltage family protocol is illustrated. c, d, Same as a, b, but from a wild-type TRAAK patch. e, f, Current response (upper) to pressure application (lower) at 0 mV from the same TRAAK(I159C, R284C) patch during perfusion of reducing (e) or oxidizing (f) bath solution. g, h, Same as e, f, but from a wild-type TRAAK patch. Scale is shown between each pair of recordings in reducing and oxidizing bath solutions.
Extended Data Figure 4 Basal activity and tension activation of TRAAK.
a, Whole cell current from a TRAAK-expressing cell during a voltage step protocol in a tenfold gradient of [K+] (EK+ = −59 mV, holding voltage = −80 mV, ΔV = 10 mV, indicated steps shown). Red dashed line indicates zero current level. b, Current–voltage relationship from experiment in a. c, Currents (upper traces) recorded from an outside-out patch excised from the same cell as in a, b. The voltage protocol in a was used with an additional pressure step (lower trace) during each voltage step. d, Current–voltage relationship from data in b (mean current 5 min after patch excision before pressure (red) and peak current during pressure step (grey)) and a recording immediately after pulling the patch (red dashes). The excised patch contains < 1% of the whole cell membrane area, but gives ∼25% of the whole cell current before and similar current during a pressure step. This is explained by very low basal activity of TRAAK with near-zero membrane tension (whole cell) and channel activation by increasing membrane tension over a broad range (intermediate tension in an excised patch to high tension in a pressurized patch).
Extended Data Figure 5 Detailed view of TM2–TM3 rotation in TRAAK.
Stereo view from the cytoplasm of an overlay of non-conductive (red) and conductive TM2–TM3 rotated (blue) conformations. Amino acids that sterically prevent TM2–TM3 rotation when TM4 is down are shown as sticks. TM2–TM3 rotates about hinges at positions G169 and G205. This rotation can only occur if TM4 is up because amino acids L172, F201 and G205 on TM2–TM3 shift (0.75–2.1 Å) to a position that would sterically clash with amino acids Y271 and V275 on TM4 in a down conformation. Translation of Y271 and V275 3.1–4.1 Å in TM4 up conformations creates space for the TM2–TM3 rotation.
Rights and permissions
About this article
Cite this article
Brohawn, S., Campbell, E. & MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 516, 126–130 (2014). https://doi.org/10.1038/nature14013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14013
This article is cited by
-
A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity
Nature Communications (2023)
-
Structural basis for assembly and lipid-mediated gating of LRRC8A:C volume-regulated anion channels
Nature Structural & Molecular Biology (2023)
-
Conformational plasticity of NaK2K and TREK2 potassium channel selectivity filters
Nature Communications (2023)
-
Mechanisms of endothelial flow sensing
Nature Cardiovascular Research (2023)
-
Mechanosensitive aquaporins
Biophysical Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.