Abstract
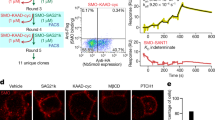
Oxysterols are a class of endogenous signaling molecules that can activate the Hedgehog pathway, which has critical roles in development, regeneration and cancer. However, it has been unclear how oxysterols influence Hedgehog signaling, including whether their effects are mediated through a protein target or indirectly through effects on membrane properties. To answer this question, we synthesized the enantiomer and an epimer of the most potent oxysterol, 20(S)-hydroxycholesterol. Using these molecules, we show that the effects of oxysterols on Hedgehog signaling are exquisitely stereoselective, consistent with the hypothesis that they function through a specific protein target. We present several lines of evidence that this protein target is the seven-pass transmembrane protein Smoothened, a major drug target in oncology. Our work suggests that these enigmatic sterols, which have multiple effects on cell physiology, may act as ligands for signaling receptors and provides a generally applicable framework for probing sterol signaling mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Varjosalo, M. & Taipale, J. Hedgehog: functions and mechanisms. Genes Dev. 22, 2454–2472 (2008).
Murone, M., Rosenthal, A. & de Sauvage, F.J. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr. Biol. 9, 76–84 (1999).
Stone, D.M. et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384, 129–134 (1996).
Marigo, V. et al. Biochemical evidence that patched is the Hedgehog receptor. Nature 384, 176–179 (1996).
Barakat, M.T., Humke, E.W. & Scott, M.P. Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol. Med. 16, 337–348 (2010).
Cooper, M.K., Porter, J.A., Young, K.E. & Beachy, P.A. Teratogen-mediated inhibited of target tissue response to Shh signaling. Science 280, 1603–1607 (1998).
Chen, J.K. et al. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743–2748 (2002).
Heretsch, P., Tzagkaroulaki, L. & Giannis, A. Cyclopamine and hedgehog signaling: chemistry, biology, medical perspectives. Angew. Chem. Int. Ed. Engl. 49, 3418–3427 (2010).
Chen, J.K. et al. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99, 14071–14076 (2002).
Sinha, S. & Chen, J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2, 29–30 (2006).
Romer, J.T. et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1+/−p53−/− mice. Cancer Cell 6, 229–240 (2004).
Corcoran, R.B. & Scott, M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. USA 103, 8408–8413 (2006).
Dwyer, J.R. et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 282, 8959–8968 (2007).
Johnson, J.S. et al. Novel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivo. J. Cell Biochem. 112, 1673–1684 (2011).
Rohatgi, R., Milenkovic, L. & Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376 (2007).
Corbit, K.C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).
LeBlanc, M.A. & McMaster, C.R. Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. J. Biol. Chem. 285, 33875–33884 (2010).
Radhakrishnan, A. et al. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 104, 6511–6518 (2007).
Janowski, B.A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. USA 96, 266–271 (1999).
Chen, W. et al. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 5, 73–79 (2007).
Hannedouche, S. et al. Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527 (2011).
Liu, C. et al. Oxysterols direct B-cell migration through EBI2. Nature 475, 519–523 (2011).
Panini, S.R. & Sinensky, M.S. Mechanisms of oxysterol-induced apoptosis. Curr. Opin. Lipidol. 12, 529–533 (2001).
Park, K. & Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 88, 1081–1087 (2010).
Theunissen, J.J. et al. Membrane properties of oxysterols. Interfacial orientation, influence on membrane permeability and redistribution between membranes. Biochim. Biophys. Acta 860, 66–74 (1986).
Rentero, C. et al. Functional implications of plasma membrane condensation for T cell activation. PLoS ONE 3, e2262 (2008).
Olkkonen, V.M. & Hynynen, R. Interactions of oxysterols with membranes and proteins. Mol. Aspects Med. 30, 123–133 (2009).
Sasaki, H. et al. A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 1997, 1313–1322 (1997).
Infante, R.E. et al. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 283, 1052–1063 (2008).
Massey, J.B. & Pownall, H.J. Structures of biologically active oxysterols determine their differential effects on phospholipid membranes. Biochemistry 45, 10747–10758 (2006).
Covey, D.F. ent-Steroids: novel tools for studies of signaling pathways. Steroids 74, 577–585 (2009).
Mannock, D.A. et al. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys. J. 84, 1038–1046 (2003).
Westover, E.J. et al. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J. Biol. Chem. 278, 51125–51133 (2003).
Gale, S.E. et al. Side chain oxygenated cholesterol regulates cellular cholesterol homeostasis through direct sterol-membrane interactions. J. Biol. Chem. 284, 1755–1764 (2009).
Rominger, C.M. et al. Evidence for allosteric interactions of antagonist binding to the smoothened receptor. J. Pharmacol. Exp. Ther. 329, 995–1005 (2009).
Kenakin, T.P. A Pharmacology Primer. 3rd edn. 101–147 (Elsevier, 2009).
Kim, J. et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 17, 388–399 (2010).
Fitzgerald, J.B. et al. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2, 458–466 (2006).
Rohatgi, R. et al. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc. Natl. Acad. Sci. USA 106, 3196–3201 (2009).
Töröcsik, D., Szanto, A. & Nagy, L. Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol. Aspects Med. 30, 134–152 (2009).
Brown, A.J. Cholesterol, statins and cancer. Clin. Exp. Pharmacol. Physiol. 34, 135–141 (2007).
Cooper, M.K. et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 33, 508–513 (2003).
Lin, Y.Y., Welch, M. & Lieberman, S. The detection of 20S-hydroxycholesterol in extracts of rat brains and human placenta by a gas chromatograph/mass spectrometry technique. J. Steroid Biochem. Mol. Biol. 85, 57–61 (2003).
Mijares, A. et al. Studies on the C20 epimers of 20-hydroxycholesterol. J. Org. Chem. 32, 810–812 (1967).
Ruprecht, J.J. et al. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 23, 3609–3620 (2004).
Cherezov, V. et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Roberts, K.D., Bandy, L. & Lieberman, S. The occurrence and metabolism of 20 alpha-hydroxycholesterol in bovine adrenal preparations. Biochemistry 8, 1259–1270 (1969).
Lütjohann, D. et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA 93, 9799–9804 (1996).
Acknowledgements
We thank members of the Rohatgi lab for helpful discussions, G. Luchetti for help with ligand affinity chromatography, P. Niewiadomski and A. Lebensohn for critical reading of the manuscript, E. Lee (Vanderbilt University) for Wnt-L cells, K. Mykytyn (Ohio State University) for the SSTR3-GFP and HTR6-GFP constructs, A. Sweet-Cordero for use of a Li-Cor Odyssey imager and M. Scott for support and use of a confocal microscope. MS analysis was conducted at the NIH – National Center for Research Resources MS facility at Washington University, supported by the NIH (RR00954, DK020579, DK056341). This work was supported by a Pew Scholar Award and an Innovation Research Grant from the Stand Up to Cancer – American Association for Cancer Research Foundation to R.R., by NIH grants to D.F.C. (GM47969 and HL67773) and P.H.S. (HL67773), and by NIH training grants to S.N. (5 T32 GM007276) and L.K.M. (5 T32 HL007275).
Author information
Authors and Affiliations
Contributions
L.K.M., K.K. and D.F.C. designed and synthesized the oxysterol analogs. S.N. performed cellular experiments with oxysterols. J.R. and P.H.S. designed and performed vesicle expansion experiments. All authors analyzed the data and contributed to the manuscript. S.N. and R.R. wrote the paper with input from L.K.M., D.F.C., J.R. and P.H.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 4954 kb)
Rights and permissions
About this article
Cite this article
Nachtergaele, S., Mydock, L., Krishnan, K. et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol 8, 211–220 (2012). https://doi.org/10.1038/nchembio.765
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.765
This article is cited by
-
Non-canonical non-genomic morphogen signaling in anucleate platelets: a critical determinant of prothrombotic function in circulation
Cell Communication and Signaling (2024)
-
Sonic Hedgehog Signaling Pathway: A Role in Pain Processing
Neurochemical Research (2023)
-
Cholesterylation of Smoothened is a calcium-accelerated autoreaction involving an intramolecular ester intermediate
Cell Research (2022)
-
High-fat diet intake ameliorates the expression of hedgehog signaling pathway in adult rat liver
Molecular Biology Reports (2022)
-
A proteome-wide map of 20(S)-hydroxycholesterol interactors in cell membranes
Nature Chemical Biology (2021)