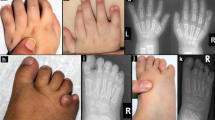
Abstract
Focal dermal hypoplasia is an X-linked dominant disorder characterized by patchy hypoplastic skin and digital, ocular and dental malformations. We used array comparative genomic hybridization to identify a 219-kb deletion in Xp11.23 in two affected females. We sequenced genes in this region and found heterozygous and mosaic mutations in PORCN in other affected females and males, respectively. PORCN encodes the human homolog of Drosophila melanogaster porcupine, an endoplasmic reticulum protein involved in secretion of Wnt proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hall, E.H. & Terezhalmy, G.T. J. Am. Acad. Dermatol. 9, 443–451 (1983).
Goltz, R.W. Arch. Dermatol. 128, 1108–1111 (1992).
Van den Veyver, I.B. Cytogenet. Genome Res. 99, 289–296 (2002).
Gorski, J.L. Am. J. Med. Genet. 40, 332–337 (1991).
Froyen, G. et al. Hum. Genet. 121, 539–547 (2007).
Derry, J.M. et al. Nat. Genet. 22, 286–290 (1999).
Kato, M. et al. Hum. Mutat. 23, 147–159 (2004).
Shirahama, S. et al. Hum. Genet. 112, 78–83 (2003).
Caricasole, A., Ferraro, T., Rimland, J.M. & Terstappen, G.C. Gene 288, 147–157 (2002).
Tanaka, K., Kitagawa, Y. & Kadowaki, T. J. Biol. Chem. 277, 12816–12823 (2002).
Zhai, L., Chaturvedi, D. & Cumberledge, S. J. Biol. Chem. 279, 33220–33227 (2004).
Takada, R. et al. Dev. Cell 11, 791–801 (2006).
Lee, J.A. & Lupski, J.R. Neuron 52, 103–121 (2006).
Tanaka, K., Okabayashi, K., Asashima, M., Perrimon, N. & Kadowaki, T. Eur. J. Biochem. 267, 4300–4311 (2000).
Tsuji, T. J. Cutan. Pathol. 9, 271–281 (1982).
Acknowledgements
We thank research subjects and their families for their participation in the research and all clinicians and genetic counselors who referred patients. We thank H.Y. Zoghbi and A.L. Beaudet for critical reading and discussions during preparation of this manuscript and L. Cooper, W. Jin and A. Casillas for technical assistance. This work was funded by the National Foundation for Ectodermal Dysplasias, in part by US National Institutes of Health (NIH) grant HD051805 and by the Tissue Culture Core, Genome Analysis Core and Gene Expression Core Laboratories of the Baylor College of Medicine Mental Retardation and Developmental Disabilities Research Center (NIH grant HD024064).
Author information
Authors and Affiliations
Contributions
X.W. contributed to array CGH, mutation analysis, qPCR and cell culture. V.R.S. contributed to research subject enrollment, clinical evaluation, manuscript writing and data analysis. J.O.P.-L. referred and evaluated individual G17, critical to the identification of the gene associated with FDH. Z.Y. contributed to mutation analysis and DNA preparation. R.R. contributed to cell culture and DNA preparation. Y.-C.K. contributed to qPCR. T.N.E. coordinated research and maintained the research subject database. C.T. contributed to RNA in situ hybridization. A.P. contributed to FISH. P.F. contributed to X chromosome inactivation studies. I.B.V.d.V. oversaw all aspects of the research and contributed to manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Phenotype of an individual with FDH with an Xp11.23 deletion, and array CGH hybridization results on DNA from her parents. (PDF 63 kb)
Supplementary Fig. 2
Mutations in PORCN. (PDF 351 kb)
Supplementary Fig. 3
X chromosome inactivation plots. (PDF 71 kb)
Supplementary Fig. 4
Copy number analysis and X chromosome inactivation studies on male samples, and sequencing profiles for male G248. (PDF 117 kb)
Supplementary Fig. 5
Xp11.23 deletion in G43. (PDF 37 kb)
Supplementary Fig. 6
Expression of Porcn mRNA in mouse embryos. (PDF 165 kb)
Supplementary Table 1
Summary of reported phenotypic features. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Reid Sutton, V., Omar Peraza-Llanes, J. et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet 39, 836–838 (2007). https://doi.org/10.1038/ng2057
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng2057
This article is cited by
-
Novel insights into PORCN mutations, associated phenotypes and pathophysiological aspects
Orphanet Journal of Rare Diseases (2022)
-
Importance of complete phenotyping in prenatal whole exome sequencing
Human Genetics (2018)
-
The aquaglyceroporin AQP9 contributes to the sex-specific effects of in utero arsenic exposure on placental gene expression
Environmental Health (2017)
-
Lung disease recalling paraseptal emphysema in a patient with Goltz syndrome
Multidisciplinary Respiratory Medicine (2016)
-
Fatty acylation of Wnt proteins
Nature Chemical Biology (2016)