Abstract
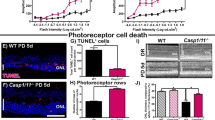
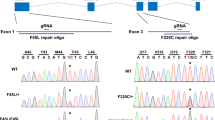
Excessive phototransduction signaling is thought to be involved in light-induced and inherited retinal degeneration. Using knockout mice with defects in rhodopsin shut-off and transducin signaling, we show that two different pathways of photoreceptor-cell apoptosis are induced by light. Bright light induces apoptosis that is independent of transducin and accompanied by induction of the transcription factor AP-1. By contrast, low light induces an apoptotic pathway that requires transducin. We also provide evidence that additional genetic factors regulate sensitivity to light-induced damage. Our use of defined mouse mutants resolves some of the complexity underlying the mechanisms that regulate susceptibility to retinal degeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Reme, C.E., Grimm, C., Hafezi, F., Marti, A. & Wenzel, A. Apoptotic cell death in retinal degenerations. Prog. Retinal Eye Res. 17, 443–464 (1998).
Chen, J., Simon, M.I., Matthes, M.T., Yasumura, D. & LaVail, M.M. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness). Invest. Ophthalmol. Vis. Sci. 40, 2978–2982 (1999).
Chen, C.K. et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl Acad. Sci. USA 96, 3718–3722 (1999).
LaVail, M.M., Gorrin, G.M., Yasamura, D. & Matthes, M.T. Increased susceptibility to constant light in nr and pcd mice with inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 40, 1020–1024 (1999).
Organisciak, D.T., Li, M., Darrow, R.M. & Farber, D.B. Photoreceptor cell damage by light in young Royal College of Surgeons rats. Curr. Eye Res. 19, 188–196 (1999).
Wang, M., Lam, T.T., Tso, M.O. & Naash, M.I. Expression of a mutant opsin gene increases the susceptibility of the retina to light damage. Vis. Neurosci. 14, 55–62 (1997).
Grimm, C. et al. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nature Genet. 25, 63–66 (2000).
Sieving, P.A. et al. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc. Natl Acad. Sci. USA 98, 1835–1840 (2001).
Wenzel, A., Reme, C.E., Williams, T.P., Hafezi, F. & Grimm, C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 21, 53–58 (2001).
Keller, C., Grimm, C., Wenzel, A., Hafezi, F. & Reme, C. Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest. Ophthalmol. Vis. Sci. 42, 476–480 (2001).
Fain, G.L. & Lisman, J.E. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: the equivalent light hypothesis. Exp. Eye Res. 57, 335–340 (1993).
Fain, G.L. & Lisman, J.E. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice. Invest. Ophthalmol. Vis. Sci. 40, 2770–2772 (1999).
Lisman, J. & Fain, G. Support for the equivalent light hypothesis for RP. Nature Med. 1, 1254–1255 (1995).
Noell, W.K. & Albrecht, R. Irreversible effects of visible light on the retina: role of vitamin A. Science 172, 76–80 (1971).
Calvert, P.D. et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc. Natl Acad. Sci. USA 97, 13913–13918 (2000).
Stryer, L. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 9, 87–119 (1986).
Rapp, L. & Williams, T. in The Effects of Constant Light on Visual Processes (eds Williams, T.P. and Baker, B.N.) 135–159 (Plenum, New York, 1980).
Rapp, L. & Williams, T. The role of ocular pigmentation in protecting against light damage. Vision Res. 20, 1127–1131 (1980).
LaVail, M.M. & Gorrin, G.M. Protection from light damage by ocular pigmentation: analysis using experimental chimeras and translocation mice. Exp. Eye Res. 44, 877–889 (1987).
Portera-Cailliau, C., Sung, C.-H., Nathans, J. & Alder, R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl Acad. Sci. USA 91, 974–978 (1994).
Chang, G.-Q., Hao, Y. & Wong, F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 11, 595–605 (1993).
Wenzel, A. et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest. Ophthalmol. Vis. Sci. 42, 1653–1659 (2001).
Wenzel, A. et al. c-fos controls the 'private pathway' of light-induced apoptosis of retinal photoreceptors. J. Neurosci. 20, 81–88 (2000).
Danciger, M. et al. A QTL on distal chromosome 3 that influences the severity of light-induced damage to mouse photoreceptors. Mamm. Genome 11, 422–427 (2000).
Redmond, T.M. et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nature Genet. 20, 344–351 (1998).
Bentrop, J. Rhodopsin mutations as the cause of retinal degeneration. Classification of degeneration phenotypes in the model system Drosophila melanogaster. Acta Anat. 162, 85–94 (1998).
Alloway, P.G., Howard, L. & Dolph, P.J. The formation of stable rhodopsin–arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 28, 129–138 (2000).
Kiselev, A. et al. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28, 139–152 (2000).
Dillon, J., Gaillard, E.R., Bilski, P., Chignell, C.F. & Reszka, K.J. The photochemistry of the retinoids as studied by steady-state and pulsed methods. Photochem. Photobiol. 63, 680–685 (1996).
Gal, A., Apfelstedt-Sylla, E., Janecke, A.R. & Zrenner, E. Rhodopsin mutations in inherited retinal dystrophies and dysfunctions. Prog. Retinal Eye Res. 16, 51–79 (1997).
Khani, S.C., Nielsen, L. & Vogt, T.M. Biochemical evidence for pathogenicity of rhodopsin kinase mutations correlated with the Oguchi form of congenital stationary night blindness. Proc. Natl Acad. Sci. USA 95, 2824–2827 (1998).
Nakazawa, M., Wada, Y. & Tamai, M. Arrestin gene mutations in autosomal recessive retinitis pigmentosa. Arch. Ophthalmol. 116, 498–501 (1998).
Yamada, T. et al. 1147delA mutation in the arrestin gene in Japanese patients with Oguchi disease. Ophthal. Genet. 20, 117–120 (1999).
Yamamoto, S., Sippel, K.C., Berson, E.L. & Dryja, T.P. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nature Genet. 15, 175–178 (1997).
Cruickshanks, K.J., Klein, R., Klein, B.E. & Nondahl, D.M. Sunlight and the 5-year incidence of early age-related maculopathy: The Beaver Dam Eye Study. Arch. Ophthalmol. 119, 246–250 (2001).
Cruickshanks, K.J., Klein, R. & Klein, B.E. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch. Ophthalmol. 111, 514–518 (1993).
Mata, N.L., Weng, J. & Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl Acad. Sci USA 97, 7154–7159 (2000).
Obin, M. et al. Calorie restriction modulates age-dependent changes in the retinas of Brown Norway rats. Mech. Ageing Dev. 114, 133–147 (2000).
LaVail, M.M., Gorrin, G.M., Repaci, M.A., Thomas, L.A. & Ginsberg, H.M. Genetic regulation of light damage to photoreceptors. Invest. Ophthalmol. Vis. Sci. 28, 1043–1048 (1987).
Hafezi, F., Marti, A., Grimm, C., Wenzel, A. & Reme, C.E. Differential DNA binding activities of the transcription factors AP-1 and Oct-1 during light-induced apoptosis of photoreceptors. Vision Res. 39, 2511–2518 (1999).
Leist, M. et al. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J. Immunol. 153, 1778–1788 (1994).
Acknowledgements
We thank M. Danciger for help determining leucine/methionine polymorphism in Rpe65; D. Brunelle for help with graphics and manuscript preparation; F. Celestin of the SCOR in Ischemic Heart Disease Histology Core for assistance with histological sections; and D. Greuter, C. Imsand and G. Hoegger for technical assistance. Support for this research was provided by the US National Institutes of Health (J.L., M.I.S. and M.S.O.), Foundation Fighting Blindness (J.L.), Massachusetts Lions Eye Research Fund (an institutional grant to the Department of Ophthalmology, New England Medical Center) and Research to Prevent Blindness Special Scholar's Award (J.L.). C.E.R. received support from the Swiss National Science Foundation, German Research Council and Velux Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hao, W., Wenzel, A., Obin, M. et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet 32, 254–260 (2002). https://doi.org/10.1038/ng984
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng984
This article is cited by
-
Tracking distinct microglia subpopulations with photoconvertible Dendra2 in vivo
Journal of Neuroinflammation (2021)
-
High dose expression of heme oxigenase-1 induces retinal degeneration through ER stress-related DDIT3
Molecular Neurodegeneration (2021)
-
Analysis from the perspective of cilia: the protective effect of PARP inhibitors on visual function during light-induced damage
International Ophthalmology (2020)
-
Molecular profiling of resident and infiltrating mononuclear phagocytes during rapid adult retinal degeneration using single-cell RNA sequencing
Scientific Reports (2019)
-
Sphingosine Kinase 2 Phosphorylation of FTY720 is Unnecessary for Prevention of Light-Induced Retinal Damage
Scientific Reports (2019)